Select The Compound With The Highest Lattice Energy
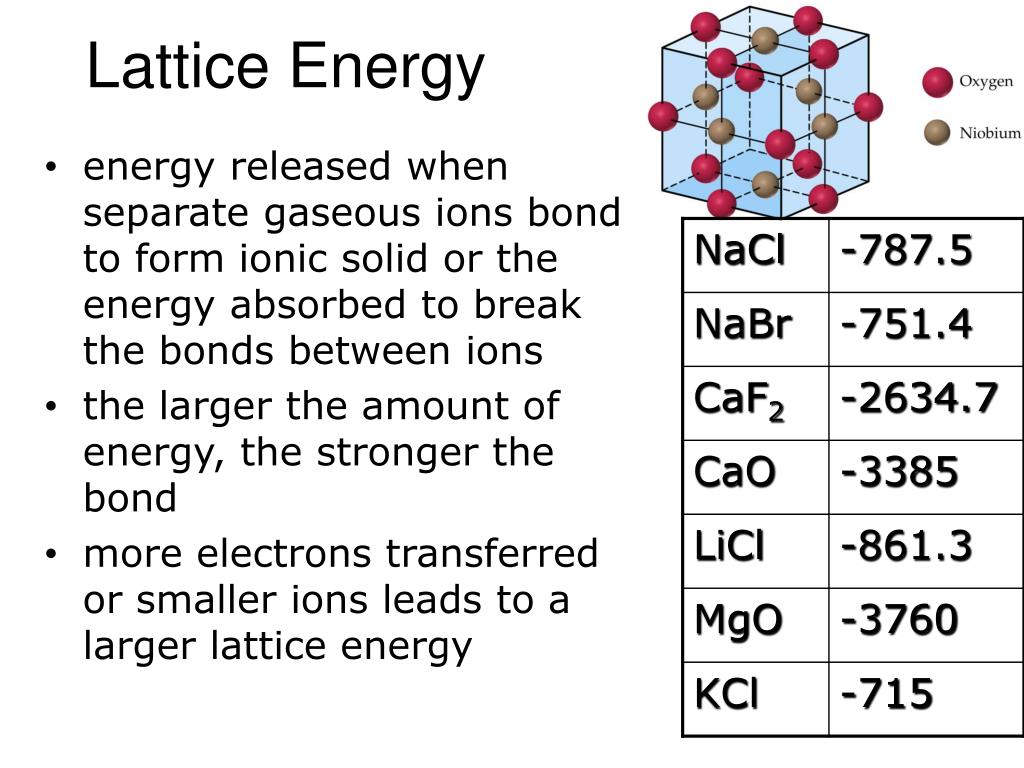
Hey there, science buddy! Ever find yourself staring at a bunch of ionic compounds and wondering, "Which one of these bad boys is gonna be the most tightly packed, the most stuck together?" Well, you're in luck, because today we're diving headfirst into the wonderfully sticky world of lattice energy. Don't worry, it sounds way scarier than it is. Think of it like a giant, cosmic superglue holding ions together. We're going to figure out how to pick the champ, the one with the highest lattice energy, like we're picking the winner of a very ionic popularity contest!
So, what exactly is lattice energy? Imagine you've got your positive ions (the cations, like little happy campers) and your negative ions (the anions, maybe a bit grumpy, who knows?). When they come together to form a crystal structure – think of it like building with LEGOs, but with charged particles – they release energy. This energy released is the lattice energy. The higher the lattice energy, the stronger those ions are holding onto each other. It’s like they’ve found their perfect ionic soulmate and are never letting go!
Why should you even care about lattice energy? Well, it’s super important for predicting a bunch of stuff. For instance, a compound with high lattice energy will generally have a higher melting point. Like, way higher. Think of trying to pry apart a super-glued model airplane versus one held together with a dab of toothpaste. The super-glued one is gonna need a lot more heat (or a really strong solvent, but we’re sticking to heat for melting points!). It also influences solubility – high lattice energy can mean a compound is less likely to dissolve because those ions are just too cozy to be bothered by pesky water molecules.
Okay, so how do we actually pick the winner? There are a couple of key factors that play musical chairs when determining lattice energy. We're not going to get bogged down in complex math formulas that would make your brain do the Macarena (unless you like that sort of thing, no judgment here!). We're going to focus on the big hitters, the things you can easily spot.
The first big hitter, and probably the most impactful, is the charge of the ions involved. Think about magnets. If you have two weak magnets, they’ll stick, but not with a death grip, right? But if you have two super-strong, industrial-strength magnets, they’ll practically fuse together. It’s the same principle with ions. The higher the charges on the ions, the stronger the electrostatic attraction between them. And a stronger attraction means a higher lattice energy. Makes sense, doesn't it?
Let’s illustrate with a classic example. Compare Sodium Chloride (NaCl) and Magnesium Oxide (MgO). Sodium (Na) has a +1 charge, and Chlorine (Cl) has a -1 charge. Magnesium (Mg) has a +2 charge, and Oxygen (O) has a -2 charge. See the difference? MgO has ions with double the charge! This means the attraction between Mg²⁺ and O²⁻ is going to be way, way stronger than the attraction between Na⁺ and Cl⁻. So, between NaCl and MgO, MgO will have the significantly higher lattice energy. It’s like the difference between a friendly handshake and a full-on, bear hug of ionic bonding!
Here’s a little mnemonic device to help you remember: "Charges charge up the lattice energy!". Say it with me! Charges charge up the lattice energy! It’s catchy, right? You can even sing it to the tune of your favorite pop song.
The second big hitter is the size of the ions. Now, this one's a little more nuanced, but still pretty straightforward. Generally speaking, the smaller the ions, the closer they can get to each other. And when charged particles are closer together, their attraction is stronger. Think about holding two really tiny magnets versus two really huge ones. The tiny ones, if they're strong enough, can get into super close contact, maximizing that magnetic pull. So, smaller ionic radius leads to higher lattice energy.
Let’s take another peek at our friends. Consider Lithium Fluoride (LiF) and Potassium Bromide (KBr). Lithium (Li) and Fluoride (F) are relatively small ions. Potassium (K) and Bromide (Br) are quite a bit larger. Even though LiF and KBr both have +1 and -1 charges (so the charge factor is equal), the LiF will have a higher lattice energy because its ions are smaller and can get closer together, creating a more intense electrostatic hug.
It’s important to remember that sometimes these factors are working together, and sometimes they're working against each other. When you're comparing, say, a compound with high charges but large ions versus a compound with low charges but small ions, you need to think about which factor is more influential. As a general rule of thumb, charge has a bigger impact than size. So, if you have to pick one to prioritize, go with the charge!

Let’s try a few more practice rounds, just for fun. Imagine you have to pick between Calcium Sulfide (CaS) and Sodium Bromide (NaBr). What do you think?
First, let's look at the charges. Calcium (Ca) is a +2, and Sulfur (S) is a -2. So, CaS has a total charge product of 2 x 2 = 4. Sodium (Na) is +1, and Bromine (Br) is -1. So, NaBr has a total charge product of 1 x 1 = 1. Whoa, CaS has much higher charges!
Now, let's think about size. Calcium is bigger than Sodium, and Sulfur is bigger than Bromine. So, the ions in NaBr are generally smaller than the ions in CaS. Hmm, so size is pushing NaBr towards higher lattice energy, and CaS towards lower.

But remember our golden rule: charge is king! The huge difference in charges between CaS (+2/-2) and NaBr (+1/-1) is going to be the deciding factor. Therefore, Calcium Sulfide (CaS) will have the higher lattice energy, hands down.
Let’s try another one. How about Lithium Chloride (LiCl) versus Potassium Chloride (KCl)?
Charges first: Li is +1, Cl is -1. K is +1, Cl is -1. The charges are the same for both compounds. So, charge isn't going to help us differentiate here. It's a tie on the charge front!
Okay, time to bring in the size factor. Lithium (Li) is a very small ion. Potassium (K) is a significantly larger ion. Since smaller ions lead to higher lattice energy, and Li is smaller than K, then Lithium Chloride (LiCl) will have the higher lattice energy.

It's like a detective story for ions! You gather clues (charges and sizes) and then use your detective skills (understanding the rules) to crack the case!
So, to recap our super-easy guide to picking the lattice energy champion:
- Look at the charges: Higher charges = stronger attraction = higher lattice energy. This is usually your biggest clue!
- Consider the sizes: Smaller ions = closer together = stronger attraction = higher lattice energy. This is your secondary clue.
- Prioritize charge: If charges are different, that's likely your winner. If charges are the same, then size becomes the main differentiator.
It’s not rocket science, is it? It’s more like ionic LEGO building with a bit of magnetic charm. You’ve got this! Every time you see a bunch of ionic compounds, you can now confidently point to the one with the most intense ionic embrace. You're practically an ionic energy guru now!
Remember, understanding these fundamental concepts in chemistry isn't about memorizing endless facts. It's about understanding the underlying principles that govern how the world around us sticks together (or doesn't!). It's about seeing the patterns, the logic, and the beautiful dance of atoms and ions. So, the next time you're presented with a lattice energy puzzle, approach it with a smile, a bit of curiosity, and the knowledge that you've got the tools to solve it. Keep exploring, keep learning, and most importantly, keep that wonderful, scientifically curious smile on your face. You're doing great!
