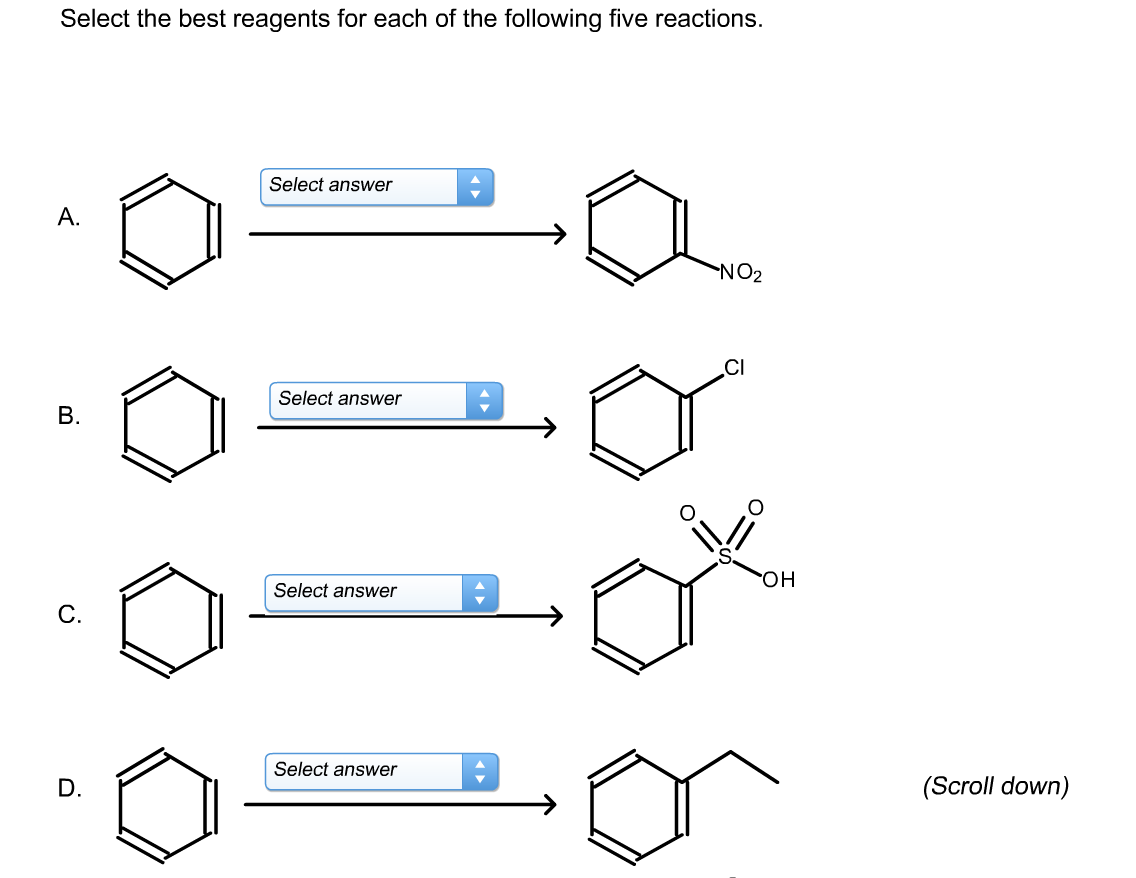
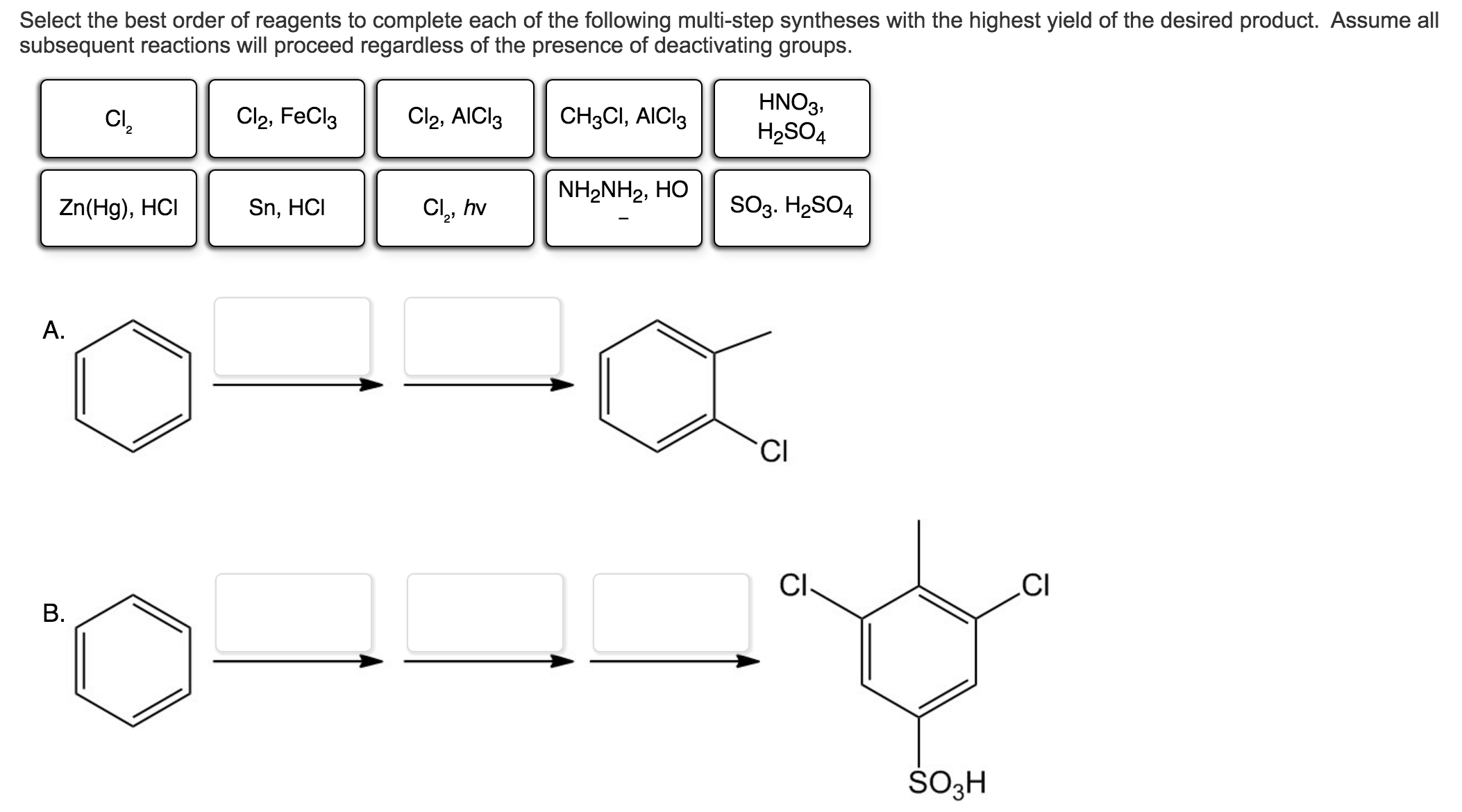
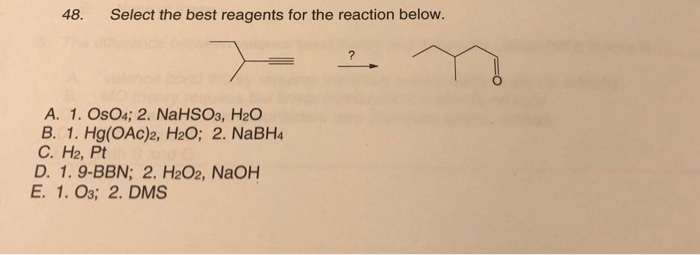
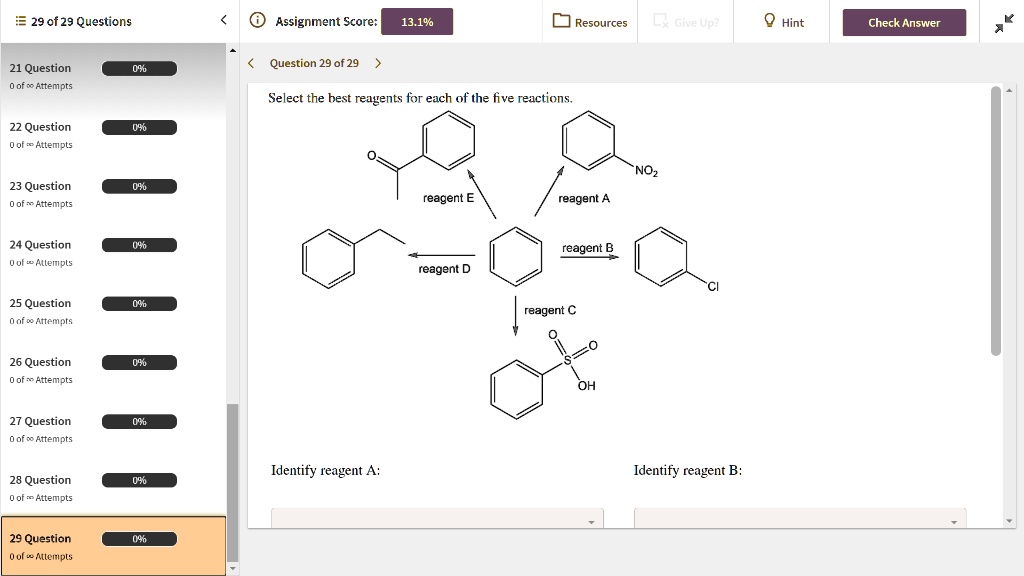
Select The Best Reagents For Each Of The Five Reactions.
Ever found yourself staring at a chemical equation and thinking, "Whoa, how do we even make that happen?" It’s kind of like trying to bake a fancy cake. You can’t just throw all the ingredients in the bowl willy-nilly, right? You need the right flour, the perfect amount of sugar, and a dash of something special to make it sing. In the world of chemistry, those "something specials" are called reagents.
And choosing the best reagents? Oh boy, that’s where the real magic happens. It’s not just about making a reaction go; it’s about making it go efficiently, safely, and sometimes, in a way that’s downright elegant. Think of it like picking the right tools for a craftsman. You wouldn't try to hammer a nail with a screwdriver, would you? So, let’s dive into five fascinating scenarios and see how chemists pick their star reagents.
When You Just Gotta Get Rid of a Halogen
So, picture this: you've got a molecule, and it's got this annoying halogen atom (like chlorine or bromine) hanging around. And you're like, "Nope, don't need you here!" What's the best way to make it say 'see ya later'? Well, depending on what you're trying to do with the rest of the molecule, you’ve got a few cool options.
If you want to swap that halogen for something else, like a nice hydroxyl group (that's an -OH group, for the uninitiated), you might reach for a strong base. Think of a strong base as a very enthusiastic cleaner that can grab onto that halogen and pull it right off, making space for the new group. It's like politely (or maybe not so politely!) asking someone to move aside so you can put something better in their spot.
But what if you want to add something else in its place? Maybe you want to link up with another molecule? Then you might consider a reagent that can act as a sort of "hook." For instance, if you have an alkyl halide (that's a carbon chain with a halogen on it), and you want to form a new carbon-carbon bond, you might turn to something like a Grignard reagent. These guys are super handy because they’re basically carbon atoms with a strong negative charge, ready to bond with almost anything!
The key here is understanding the mechanism – how the reaction actually happens step-by-step. Are we kicking the halogen out directly? Or is it a more involved dance? The reagent you choose dictates the entire dance routine. Pretty neat, huh?
Making Things Shiny and New: Oxidation Reactions
Oxidation. It sounds a bit intense, doesn’t it? Like something super aggressive. But in chemistry, it’s often about making things better, cleaner, or ready for the next step. Think of it as giving a molecule a little polish, or maybe even a complete makeover.
One of the most common tasks is oxidizing alcohols. You know, those molecules with an -OH group? Depending on the alcohol and what you want to turn it into, you’ve got a whole toolbox of oxidizing agents. If you just want to nudge it from an alcohol to an aldehyde (one step up the ladder), you might use a milder oxidant. Something like PCC (pyridinium chlorochromate) is a popular choice. It’s effective without being overly aggressive, like a gentle buffing cloth.
But if you're aiming for a carboxylic acid (that's like a fancier, more acidic version of an alcohol), you're going to need something with a bit more oomph. Potassium permanganate (KMnO4) or chromic acid are the heavy hitters here. These are like the industrial-strength polishers that can really get the job done, transforming that alcohol into something completely different.
Why is this cool? Because oxidation is everywhere! It’s how our bodies get energy from food, and it’s crucial in making plastics, medicines, and all sorts of materials we use every day. Picking the right oxidant is like choosing between a gentle scrub and a power wash – it completely changes the outcome.
The Art of Reduction: Taking Things Down a Notch
If oxidation is about adding oxygen or electrons, reduction is its opposite: taking them away. It's like deflating a balloon – taking something away to make it smaller or less reactive. And just like with oxidation, there are some seriously cool reagents for the job.
A classic task is reducing aldehydes and ketones (molecules with a carbonyl group, C=O) back into alcohols. For this, we often turn to metal hydrides. The most famous ones are probably lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4). LiAlH4 is the super-powered, no-holds-barred option. It's incredibly reactive and can reduce pretty much any carbonyl group. You have to be a bit careful with it, though, as it reacts violently with water – think of it as a really strong, slightly temperamental chef.
NaBH4, on the other hand, is a bit more of a gentle soul. It's selective and will happily reduce aldehydes and ketones, but it’s less reactive with other functional groups. It’s like a sous chef who’s great at specific tasks but doesn’t go around rearranging the whole kitchen. This selectivity is a huge deal in complex molecules, where you might only want to change one part without messing up the rest.
Reducing nitro groups (NO2) to amines (NH2) is another common transformation. This is vital for making dyes and pharmaceuticals. Catalytic hydrogenation, using hydrogen gas (H2) with a metal catalyst like palladium or platinum, is a super efficient way to do this. It's like having a silent partner that quietly helps things get done.
Building Bigger Molecules: Carbon-Carbon Bond Formation
Okay, now things get really exciting. This is where chemists play with LEGOs, but on a molecular level. The ability to connect carbon atoms together to build larger, more complex structures is fundamental to creating pretty much everything organic. So, what are the go-to reagents for this molecular construction project?
We already touched on Grignard reagents for this. They’re like little carbon nucleophiles that can attack electrophilic carbon centers, forming a new bond. It's like having a tiny, eager hand ready to grab onto another molecule and stick them together.
Another superstar is the Wittig reagent. This one is specifically designed to convert carbonyl compounds (aldehydes and ketones) into alkenes (molecules with a carbon-carbon double bond). Imagine you have a C=O group and you want to turn it into a C=C group. The Wittig reagent is the specialist for that exact job. It’s like a specialized tool that performs a very specific, but incredibly useful, transformation.
And let's not forget the Suzuki coupling. This is a more modern marvel, involving palladium catalysts. It's incredibly versatile and allows chemists to join together different types of organic fragments, often with amazing precision. It’s like having a master architect who can seamlessly connect different building blocks to create something truly magnificent.
The beauty of these reactions is that they allow us to build molecules from the ground up, creating everything from life-saving drugs to the materials that make our world go round.
Making Things Stable: Protecting Groups
Sometimes, when you’re working on a complex molecule, you need to perform a reaction on one part of it without affecting another part that’s a bit more sensitive. It’s like performing surgery – you need to protect the healthy tissue while you operate on the damaged part. In chemistry, we use something called protecting groups.
These are temporary chemical modifications that we add to a reactive functional group to make it less reactive, essentially "protecting" it. Once the desired reaction is done elsewhere on the molecule, we can easily remove the protecting group, revealing the original functional group.
For example, if you have an alcohol (-OH) and you want to do a reaction that would mess with that alcohol, you can convert it into something like a silyl ether. This "protects" the alcohol. Common silylating agents include TMSCl (trimethylsilyl chloride) or TBDMSCl (tert-butyldimethylsilyl chloride). These reagents are like putting a temporary "do not disturb" sign on the functional group.
Once you're done with your other reactions, you can use a different reagent, like a fluoride ion source or an acid, to "deprotect" the alcohol and get it back to its original form. It’s a clever strategy that allows chemists to perform multiple steps on a single molecule with incredible control.
So, you see, selecting the right reagent is more than just picking a chemical. It’s about understanding the nuances of molecular behavior, the desired outcome, and the most elegant and effective way to get there. It’s a constant dance between knowledge, intuition, and a little bit of chemical artistry!
