Select The Atomic Models That Belong To The Same Element.

Hey there, science curious buddies! Ever look at a bunch of tiny drawings that are supposed to be atoms and think, "Uh, are these all the same thing, or am I just seeing fuzzy dots?" Well, buckle up, buttercup, because we're diving into the super-duper fun world of selecting atomic models that belong to the same element. It’s like a cosmic game of I Spy, but with protons and neutrons!
Imagine atoms as little LEGO bricks of the universe. They're the building blocks for… well, everything. You, me, that slightly questionable donut in your hand – all made of atoms. And just like LEGO bricks come in different colors and sizes, atoms can look a bit different even if they're technically the same kind of elemental LEGO.
So, what makes an atom truly an atom of, say, carbon? It's not its swirly electron cloud. It’s not even how many neutrons are crammed in its tiny nucleus. Nope! The real VIP, the true identity badge of an atom, is its number of protons. Think of protons as the atom's social security number. It’s unique and it never changes for that element.
The Proton Powerhouse!
Every element has a specific number of protons. Hydrogen? Always has one proton. Helium? Always two. Lithium? Three. You get the picture. This number is called the atomic number, and it’s the key to unlocking the mystery.
So, when you see a bunch of atomic models, and you’re asked to pick the ones for the same element, you’re basically looking for the ones with the same number of protons. Easy peasy, right?
But here’s where things get a little quirky and super interesting. What if the atoms of the same element have a different number of neutrons? Are they still the same element? Drumroll, please… YES!
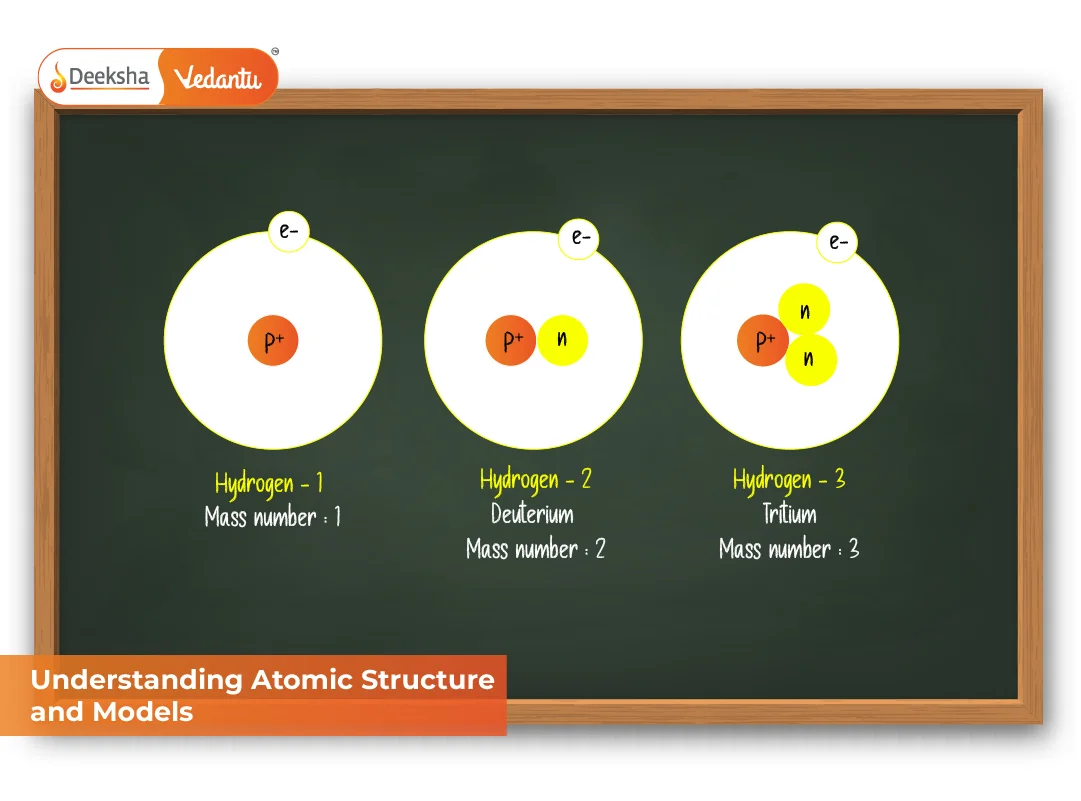
Meet the Isotopes: The Element's Cousins!
This is where our fun little game gets a twist. Atoms of the same element that have a different number of neutrons are called isotopes. They're like siblings, or maybe really close cousins, to the main atomic family. They’ve got the same number of protons (that’s what makes them the same element), but their neutron count is a bit different.
Why does this matter? Well, it’s kind of like having two identical twins, but one is slightly more muscular. They’re still the same person, but they might have different strengths. Neutrons add to the mass of an atom, so isotopes of the same element will have slightly different masses. It's a subtle difference, but it can have a big impact in the real world!
Think about carbon. The most common form of carbon, the one that makes up, you know, us, has 6 protons and 6 neutrons. That’s carbon-12. But there’s also carbon-13 (6 protons, 7 neutrons) and carbon-14 (6 protons, 8 neutrons).
Carbon-14 is super famous. It's used in radiocarbon dating. This is where science gets seriously cool! Scientists can use the amount of carbon-14 left in ancient artifacts to figure out how old they are. How neat is that? A quirky atom with an extra neutron helping us unearth ancient mysteries!

Visualizing the Atomic Zoo!
When you’re looking at atomic models, you’ll often see simplified representations. You might see circles representing protons, neutrons, and electrons. Sometimes, it's just a number in the center representing the nucleus.
So, if you’re presented with a bunch of these models, your mission is to find the ones where the number of protons (the identity card!) is identical. Don't get sidetracked by the number of electrons (those are the flighty ones that can change!) or the neutrons (the slightly bulkier, but still family, members).
Let’s say you see a model with: * 3 protons, 3 neutrons, 3 electrons. That’s Lithium! * Another one with: 3 protons, 4 neutrons, 3 electrons. Still Lithium! See? Same protons, different neutrons. An isotope! * And then one with: 4 protons, 5 neutrons, 4 electrons. Hello, Beryllium! Definitely not Lithium.
Your job is to grab all the Lithium models. The one with 3 neutrons and the one with 4 neutrons are a dynamic duo, belonging to the same element. They’re siblings in the atomic family album!

Why is This Even Fun?
Okay, okay, I know what you’re thinking. "Why should I care about protons and neutrons and picking atom pictures?" Because, my friends, it’s the foundation of understanding the universe! It’s like learning your ABCs before you can read a thrilling novel.
Plus, it’s a fantastic brain teaser! It’s like a puzzle where the pieces are invisible and make up everything. And there’s a certain elegance to it, isn’t there? This idea that something as diverse and complex as our world boils down to these fundamental, tiny particles, all governed by simple rules like the number of protons.
It’s also a great way to appreciate how much science has figured out. We can see these atoms (well, not directly, but we have incredibly clever ways of studying them!), and we can classify them. It’s a testament to human curiosity and ingenuity.
And let’s be honest, who doesn’t love a good classification game? It’s like organizing your comic book collection or sorting your LEGOs by color and size. There’s a satisfying order to it.

The Quirky Side of Atoms
Did you know that most of the universe is actually made of stuff we can’t even see? Dark matter and dark energy. Atoms are the things we can see and touch, the "normal matter." And within that normal matter, elements are like different flavors of ice cream. You can have vanilla (hydrogen), chocolate (carbon), strawberry (oxygen), and all sorts of wacky combinations!
And isotopes? Some isotopes are stable, just chilling out. Others are radioactive and… well, they’re not so chill. They’re busy decaying and spitting out little bits of energy. It’s kind of like the universe’s way of recycling. Some radioactive isotopes are used in medicine, like in PET scans, to help doctors see inside your body. So, even the unstable ones have their uses!
So, next time you’re presented with a bunch of atomic models, don’t just see random blobs. See the potential for connection! See the subtle differences that make each atom unique, and the powerful similarities that bind them together as part of the same elemental family. It’s a microscopic world with a macroscopic impact, and understanding it is a seriously fun adventure.
Keep your eyes peeled for those proton counts, ignore the electron count unless you're specifically asked about ions (that's another fun topic for another day!), and let the neutron count be a reminder that even within the same family, there can be delightful variations. Happy atom hunting!
