Redox Titration Potassium Permanganate And Sodium Oxalate Lab Report
Ever feel like your science experiments are a little, well, meh? Like you're just stirring stuff and hoping for the best? Well, buckle up, buttercups, because we're about to dive into a lab adventure that's more exciting than a surprise pizza party and easier to follow than your favorite TikTok dance! Today, we're talking about the glorious world of Redox Titration, and our star players are none other than the magnificent Potassium Permanganate and the wonderfully reactive Sodium Oxalate.
Imagine you're a detective, and your mission is to figure out the exact amount of a certain ingredient in a mysterious potion. That's pretty much what we're doing here, but instead of solving a crime, we're solving a chemical puzzle! And our magnifying glass? It's a fancy tool called a burette, which is basically a super-precise droppy-tube. We’re going to use this to add one chemical to another, drop by drop, until BAM! We hit the perfect point.
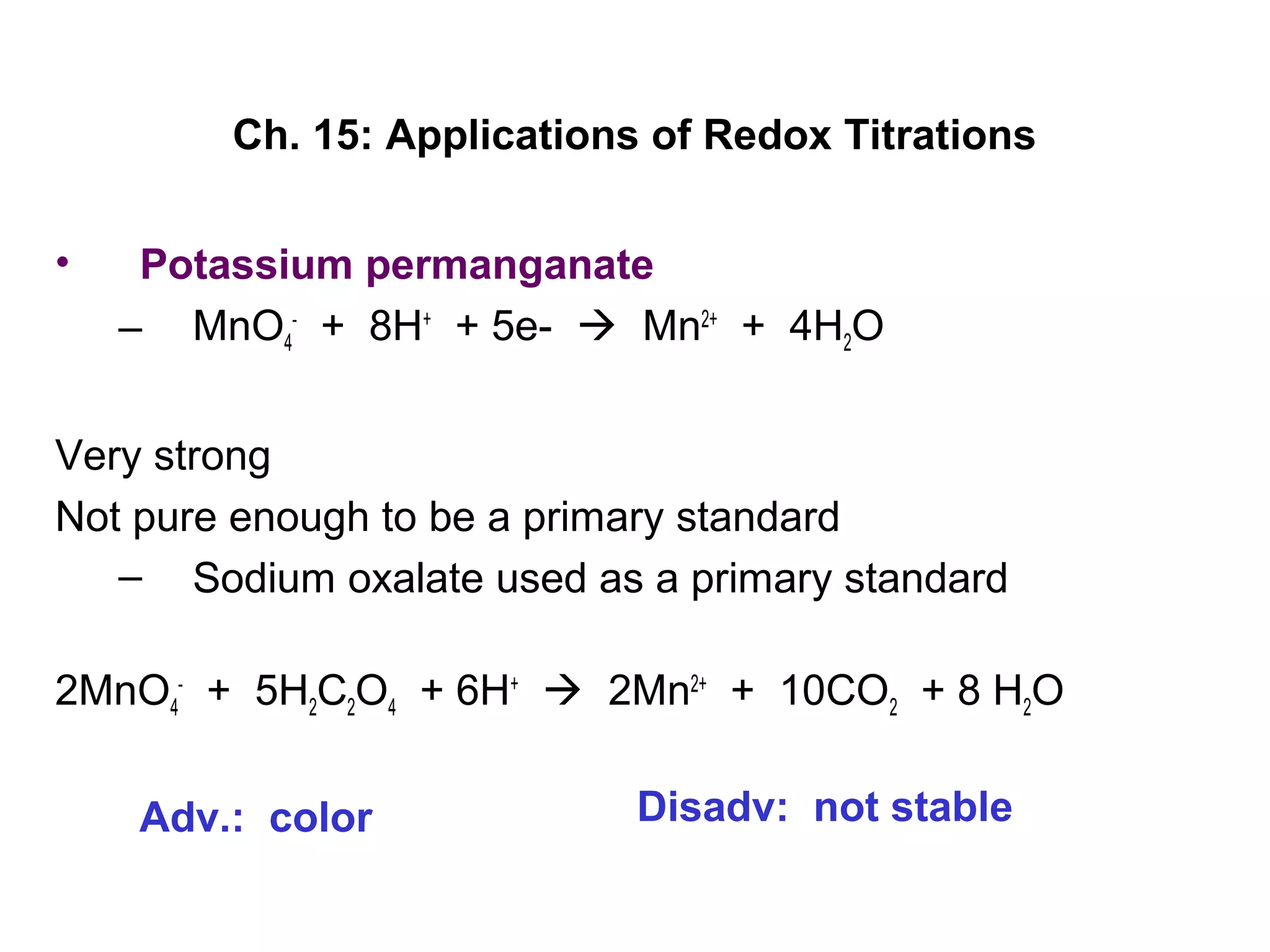
So, let's meet our contenders. First up, the dazzling diva of the lab: Potassium Permanganate. This stuff is famous for its intense purple color. It’s like the emo kid of the chemical world, but in a good way – it's got a strong personality and makes a dramatic statement! When it’s in its pure form, it's a vibrant, almost regal purple. But here’s the cool part: when it gets involved in a reaction, its color starts to fade. It’s like it's giving away its energy, its very essence, to make something new happen. It’s a true giver!
And then we have our co-star, the mild-mannered but mighty Sodium Oxalate. This one might not have the flashy purple hue, but don't let that fool you. It’s ready to rumble and play its part in our chemical drama. When Sodium Oxalate meets the enthusiastic Potassium Permanganate, sparks literally fly, in a chemical sense! They get into a tango, a dance of electrons, where one gives and the other takes. This is what we call a redox reaction. It’s a fancy term for a chemical love story where electrons are exchanged. Think of it like swapping trading cards, but way, way cooler and with more bubbling.
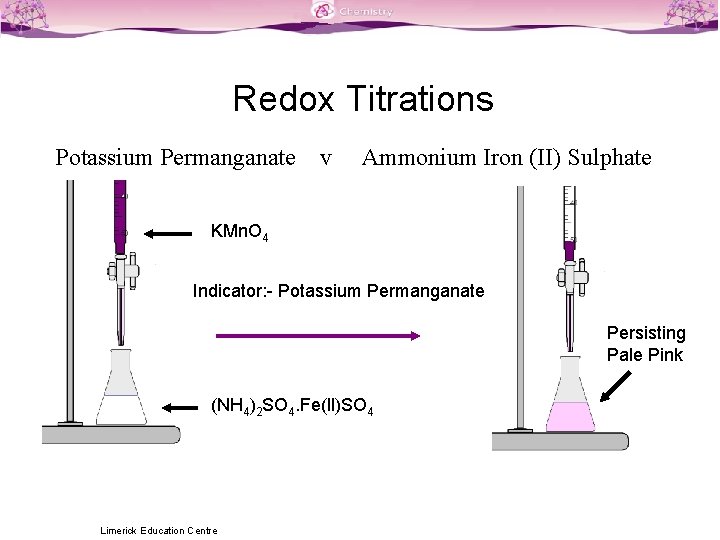
Now, how do we know when our dance is complete? This is where the magic happens, folks. We're looking for a subtle, yet unmistakable sign. As we carefully drip the Potassium Permanganate into our Sodium Oxalate solution, something amazing occurs. At first, you’ll see the purple color disappear almost instantly, like a magic trick. But then, as you get closer and closer to that "sweet spot," the purple color starts to linger. And then, just as you're thinking, "Is it happening? Is it really happening?", you add one single drop of Potassium Permanganate, and BOOM! The entire solution takes on a faint, but persistent, pinkish hue. That, my friends, is the endpoint! It's the signal that all the Sodium Oxalate has had its electron-swapping fun, and there's no more to react with. It’s like the last guest arriving at the party, and suddenly everything feels complete.
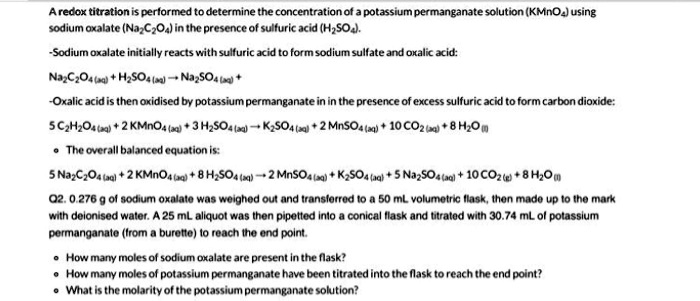
This seemingly simple color change is our golden ticket. It tells us precisely how much Potassium Permanganate we used to complete the reaction. And why is this important? Because knowing that allows us to calculate all sorts of awesome things! We can figure out the concentration of the Sodium Oxalate, or even use it to figure out the purity of other chemicals. It’s like having a secret superpower that lets you unlock the hidden secrets of your solutions!
Think of it like baking. You need the exact right amount of flour to sugar to make the perfect cookie. Too much or too little, and you’re left with a sad, flat disc or a crumbly mess. Titration is our way of making sure our chemical "recipes" are spot on!
The beauty of this experiment lies in its simplicity and its accuracy. You don't need a Ph.D. to grasp the concept, but the results can be incredibly precise. It’s a perfect example of how even a basic chemical reaction can be a powerful tool for discovery. And let’s be honest, who doesn’t love a good color change? It’s visually satisfying, like watching a beautiful sunset, but with a much more practical application!
So, next time you're in the lab, or even just thinking about chemistry, remember the dynamic duo of Potassium Permanganate and Sodium Oxalate. They might sound complicated, but they’re really just two chemicals having a very precise and colorful conversation. And we, the chemists, are just there to listen in and figure out what they’re saying. It’s a thrilling dance of electrons, a symphony of color, and a whole lot of fun. So, go forth, experiment, and let the purple magic guide you to some amazing discoveries! You’ll be a titration titan in no time!
