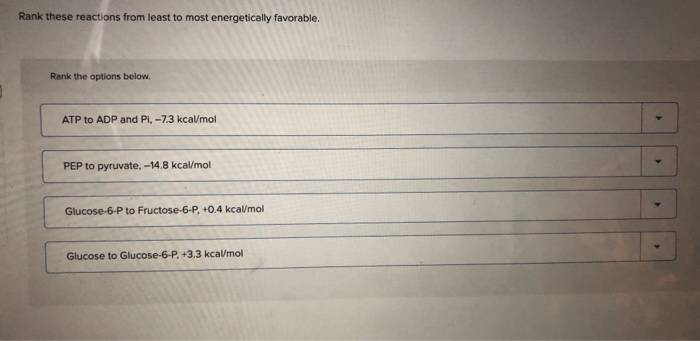
Rank These Reactions From Least To Most Energetically Favorable
Hey there, coffee buddy! Grab your mug, settle in. Today, we're diving into something that might sound a little intimidating at first glance, but trust me, it’s actually pretty cool. We're gonna rank some chemical reactions. Yeah, I know, sounds intense, right? Like something out of a super-serious science documentary. But honestly? It's more like figuring out which of your friends is the most likely to volunteer for that extra-long hike. You know? The ones who are just super gung-ho, practically vibrating with energy to get going? That's kind of what we're talking about here.
We're talking about "energetically favorable reactions." Fancy words, I know. But all it really means is, which reactions are like a toddler who just found a bag of candy – they just want to happen, and they'll get there with minimal fuss. They're the ones that give off energy, or, you know, are just really, really easy to get started. Think of it like a cozy blanket on a cold day. Some reactions are just inherently cozy. They’re stable, they’re happy where they are, and they don’t need much convincing to chill out.
So, how do we figure out this whole "energetically favorable" thing? Well, scientists have this super handy tool called Gibbs Free Energy. Don't let the name scare you. It’s basically a way to predict if a reaction will happen spontaneously. If the Gibbs Free Energy change (we usually call it ΔG, pronounced "delta G," because scientists love their abbreviations) is negative, then BAM! The reaction is like, "Yup, I'm doing this!" It's energetically favorable. If it's positive, well, that reaction is more like your teenager on a Saturday morning – you gotta really push it to get it moving. And if it's zero? It's just chilling, perfectly balanced. Not going either way unless you give it a nudge.
Now, there are a few things that play into this ΔG number. There's the enthalpy change (ΔH), which is all about whether the reaction gives off heat (exothermic, usually good for being favorable) or absorbs heat (endothermic, often not so favorable). Then there's the entropy change (ΔS), which is basically a measure of disorder or randomness. Think of your room after a good party. That's high entropy! Reactions that increase disorder are generally more favorable. And finally, there's temperature (T), because, you know, things change when you heat them up or cool them down. They’re all linked up in this equation: ΔG = ΔH - TΔS. It’s like a recipe for spontaneity!
But enough of the nitty-gritty science talk for a sec. Let’s get to the fun part: ranking! We’re going to take a few common reactions and put them in order from least energetically favorable to most energetically favorable. Think of it like lining up your dog’s toys from the one he ignores to the one he’s absolutely obsessed with. It’s a spectrum, and we’re going to navigate it!
Okay, Let's Get Our Reactions Lined Up!
For our little ranking party today, we’re going to look at:
- Photosynthesis (Yeah, the plants! They're doing their thing.)
- The Haber-Bosch process (This one's a biggie for making ammonia, super important!)
- Rusting of iron (Oh, the drama of oxidation!)
- Combustion of methane (Basically, burning natural gas. You know, for your stove or that cozy fireplace.)
- Melting of ice (Simple, everyday stuff.)
Now, these are just examples, and their "favorability" can change a tiny bit depending on the exact conditions. But we're going to look at them under pretty standard conditions. So, let's put on our thinking caps, maybe sip our coffee, and figure out who's who in the world of "go go go!" reactions.
The Least Energetically Favorable – The Hesitators
Alright, who's at the back of the line, looking a little unsure? Who needs a pep talk?
Melting of Ice (Solid Water to Liquid Water):
So, ice melting. At standard temperature and pressure, this one isn't exactly jumping to happen. Think about it: you leave an ice cube on the counter on a chilly day. Is it going to melt super fast? Probably not. It's actually endothermic, meaning it absorbs heat from its surroundings to melt. That’s a bit of a drag on favorability. Plus, going from a nice, ordered solid structure to a more jumbled liquid decreases entropy. Uh oh. So, while it does happen at temperatures above 0°C, it's not exactly a runaway train of a reaction. It needs that push of heat.
Under standard lab conditions (say, 25°C and 1 atm pressure), melting ice is actually not spontaneous. You need temperatures above the freezing point for it to be favorable. So, compared to some of the other guys we're about to meet, this is our chillest, most laid-back reaction. It’s the one that’s happy to just… be. Until you crank up the heat, of course. Then it’s like, "Okay, fine, I'll melt."
Photosynthesis:
Now, plants! They're amazing, right? Turning sunlight into food. But here's the kicker: photosynthesis itself, the actual chemical reaction, is not spontaneous. Like, at all. It requires energy input. Where does it get that energy? From sunlight! Plants are basically solar-powered factories. They use light energy to force this reaction to happen. Without that external energy boost, it's like trying to start your car by just wishing it would go. Nope. It needs a spark, or in this case, a photon.
The overall equation for photosynthesis looks something like 6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂. See that "Light Energy" on the left? That's the clue. It's an endergonic process, meaning it requires energy to proceed. So, while it's crucial for life on Earth, the chemical transformation itself isn't something that would just whip itself up in a dark lab. It's dependent on that glorious sunshine. So, it's definitely not as "go-go-go" as some of the others.
The Middle Ground – The Willing Participants
These reactions are like your friend who’s willing to help you move, but they’re not exactly leaping out of bed at 5 AM to do it. They’ll get there, maybe with a sigh, but they'll do it.

Rusting of Iron (Oxidation of Iron):
Ah, rust. The bane of many a metal object. This is a classic example of a redox reaction, where iron (Fe) reacts with oxygen (O₂) in the presence of water to form iron oxides, like Fe₂O₃·nH₂O. It’s a slow process, but it definitely happens on its own. You don't have to make iron rust; it just kinda does it over time when exposed to the elements. Pretty annoying, if you ask me, especially when you've got a nice new bike!
The enthalpy change for rust formation is generally negative, meaning it releases heat. That's a good sign for favorability. However, the entropy change can be a bit more complex. But overall, under normal atmospheric conditions, it's considered spontaneous, but it's not blazing fast. It’s like watching paint dry, but in slow motion, and with potential structural integrity issues later on. It’s happening, but it's not exactly an explosion.
It's a reaction that's thermodynamically favorable, meaning it can happen without continuous energy input. But it might take days, weeks, or even years to see significant rust formation. So, it’s not the most favorable, but it's certainly more inclined to happen than just, say, your hammer spontaneously turning into a pile of dust. That’s good to know, I guess? Less exciting than a big boom, but definitely a thing that occurs without your direct intervention.
The Haber-Bosch Process:
Okay, this one is super important for agriculture – it’s how we make ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂). And get this: it's actually exothermic, meaning it gives off heat. That’s usually a big win for favorability! However, there’s a catch. The reaction is also associated with a decrease in entropy. Why? Because you're taking two gases and turning them into one, so things are getting a bit more ordered. So, you have a trade-off: good on enthalpy, not so great on entropy.
Also, the activation energy for this reaction is super high. That means you need pretty extreme conditions – high temperatures (like, really high, 400-450°C!) and high pressures (around 150-250 atm) – to get it going fast enough. So, while the overall reaction is thermodynamically favorable at lower temperatures, it's kinetically very slow. That's why they have to use these intense conditions. They’re essentially giving it a massive kick to overcome that initial hurdle. So, it wants to happen, but it’s a bit of a diva about starting.
Think of it like this: the universe wants to make ammonia. It's a good idea, energetically speaking. But that initial bond between the nitrogen molecules is really, really strong. It's like a stubborn knot that needs a lot of force to untangle. So, while the idea of forming ammonia is favorable, the execution requires some serious effort and special equipment. It's a willing participant, but it demands a good setup. We're getting warmer in our ranking, though!
The Most Energetically Favorable – The Eager Beavers
These are your friends who are already at your door with running shoes on, asking if you're ready yet. They’re practically vibrating with enthusiasm!
Combustion of Methane:
Now we're talking! Burning methane, the main component of natural gas. This is what powers a lot of our homes and industries. And let me tell you, this reaction is enthusiastically favorable. It’s highly exothermic, meaning it releases a ton of heat. Plus, you’re going from a single molecule (methane) and oxygen to multiple smaller molecules (carbon dioxide and water), which increases the entropy. So, you’ve got a double whammy of good things happening!
The overall reaction is CH₄ + 2O₂ → CO₂ + 2H₂O. The ΔG for this is very negative. It's the kind of reaction that, once it gets started, is going to keep going as long as you feed it fuel and oxygen. It's like a happy, energetic dog that just got a new squeaky toy – it's not going to stop until it’s completely satisfied (or out of toys). It’s a classic example of a spontaneous, highly favorable reaction.

Think of lighting a gas stove. You get a spark, and poof, you have fire! It’s immediate, it releases heat, and it’s not something you have to wrestle with. It's just ready to go. This is the kind of reaction that makes you feel powerful, like you're harnessing the energy of the universe. It’s definitely at the top of our "go-go-go" list. It’s not just favorable; it's very favorable.
So, To Recap Our Energetic Lineup:
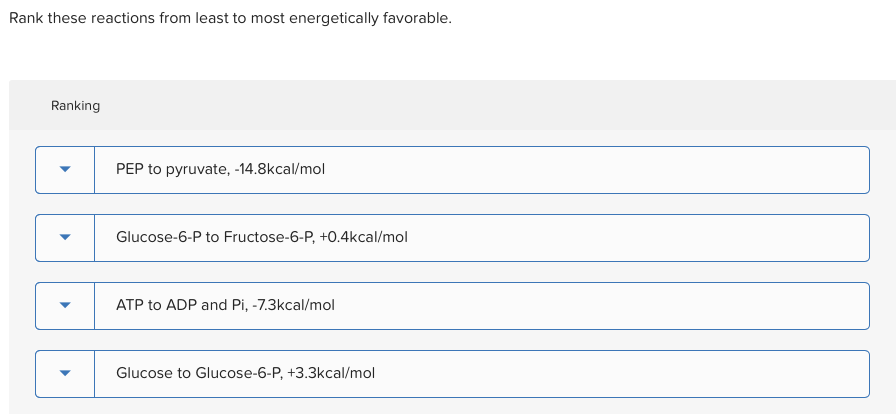
From least to most energetically favorable, under typical conditions:
- Melting of Ice (at sub-freezing temperatures): Needs a good amount of heat input and isn't naturally inclined to happen without it. It's the reluctant participant.
- Photosynthesis: Absolutely requires external energy (sunlight!) to occur. It's the solar-powered marvel that wouldn't happen otherwise.
- Rusting of Iron: Spontaneous but slow. It happens eventually, but it’s not exactly a firecracker. It's the slow and steady winner (of decay).
- The Haber-Bosch Process: Thermodynamically favorable, but requires extreme conditions to overcome the activation energy. It wants to happen, but it’s a bit of a drama queen about starting.
- Combustion of Methane: Highly exothermic and entropy-increasing. It’s the enthusiastic overachiever, ready to burst into action.
See? Not so scary, right? It's all about understanding the forces at play, and how much of a "push" or "pull" a reaction has. Some reactions are just naturally inclined to happen, releasing energy and becoming more disordered, while others need a bit more… encouragement. Or a whole lot of encouragement, in some cases!
It’s kind of like human nature, isn’t it? Some people are just go-getters, others need a little nudge, and some… well, they need a full-on intervention! Chemical reactions are no different. They have their own personalities, their own levels of enthusiasm. And by understanding their ΔG, we get a little peek into their energetic souls. Pretty neat, huh?
So, next time you see a fire, or a plant growing, or even just a rusty bike chain, you can have a little internal chat about the energetic favorability of what’s going on. You'll be like, "Ah, yes, the combustion of methane, a true paragon of spontaneity!" Or, "My, that ice is really struggling to melt at this temperature, isn't it? Such low energetic favorability!" You'll sound so smart, it'll be like you're secretly a chemistry superhero. Just don't expect people to clap. Unless, of course, you make it into an actual, energetically favorable show!
Anyway, that’s our little coffee chat about reaction rankings. Hope it was enlightening and, dare I say, a little bit fun! Now, if you’ll excuse me, I think my own spontaneous reaction to more coffee is calling my name.
