Rank These Elements According To First Ionization Energy
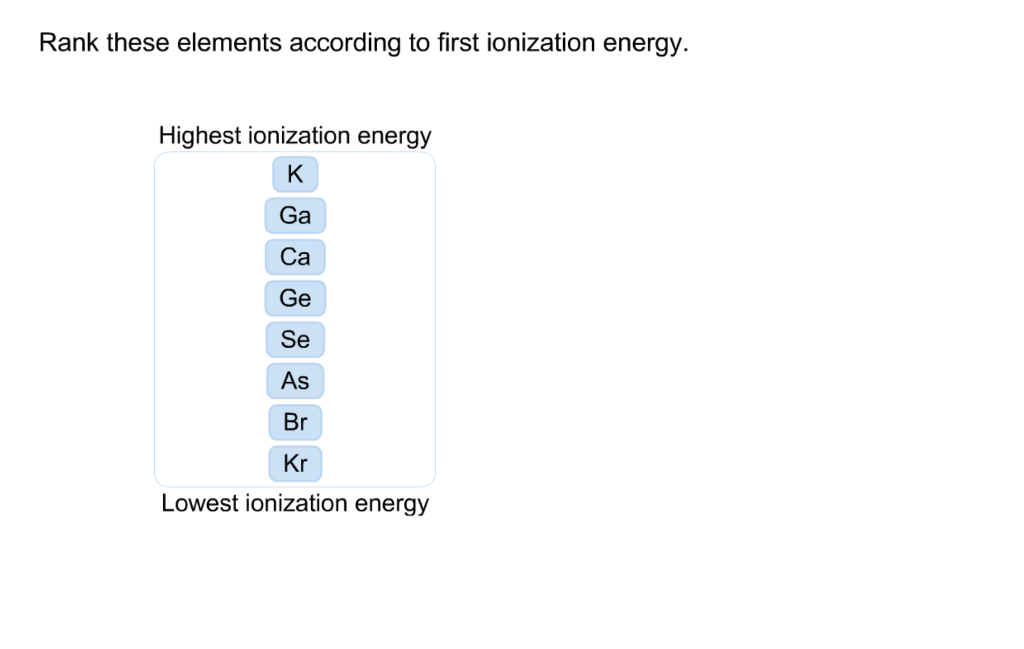
Ever feel like you're staring at a giant, colorful chart and wondering, "What's the big deal?" Well, buckle up, because we're about to dive into something that might sound a bit science-y but is actually surprisingly fun and incredibly insightful: ranking elements by their first ionization energy! Think of it like a popularity contest for atoms, but instead of who has the most followers, it's about who's the most "reluctant" to give up their electrons. This might seem niche, but understanding this concept unlocks a whole new level of appreciation for the building blocks of our universe, from the air we breathe to the stars in the sky. It's a fundamental concept that explains so much about how elements interact, form compounds, and behave in chemical reactions. Plus, for anyone who's ever tinkered with chemistry, or even just watched a cool science documentary, this is a key piece of the puzzle that makes everything else click.
Why Does This Matter?
So, what's the point of ranking these elements? It's all about understanding electron behavior. The first ionization energy is the minimum energy required to remove the outermost electron from a neutral atom in its gaseous state. Imagine an atom as a tiny solar system, with electrons orbiting the nucleus. Ionization energy is like asking, "How much energy does it take to nudge that outermost planet out of its orbit and send it flying into space?" Elements with high ionization energies hold onto their outer electrons very tightly. They're like fortress-like atoms, not eager to share or lose their precious electron. On the flip side, elements with low ionization energies are more generous, readily letting go of their outermost electron when a little nudge of energy is applied.
This "electron generosity" or "reluctance" is a huge deal. It directly dictates how elements will bond with each other. Elements that easily lose electrons tend to form positive ions (cations), while elements that readily gain electrons form negative ions (anions). This dance of electron transfer is the basis for ionic bonding, a fundamental way atoms stick together to form compounds like table salt (NaCl). For example, elements like sodium (Na), which are on the left side of the periodic table, have low ionization energies and are very happy to give up an electron to become Na+. Elements like chlorine (Cl), on the other side, have high ionization energies but a strong pull for electrons, readily accepting one to become Cl-. The opposite charges then attract, forming a stable bond.
Beyond ionic bonding, ionization energy also plays a role in covalent bonding, where electrons are shared. The difference in ionization energies between two atoms can influence how polar a covalent bond becomes. A large difference suggests one atom pulls the shared electrons much more strongly than the other, creating partial positive and negative charges within the molecule. This subtle difference can significantly affect a molecule's properties, like its solubility in water or its boiling point.
Think about the practical applications! Understanding ionization energies helps chemists predict how reactions will occur, design new materials with specific properties, and even develop catalysts that speed up industrial processes. It's a cornerstone of understanding reactivity, metallic character, and even the stability of different chemical species. So, when we rank elements by their ionization energy, we're not just playing a game; we're uncovering fundamental truths about the universe's intricate chemical language.
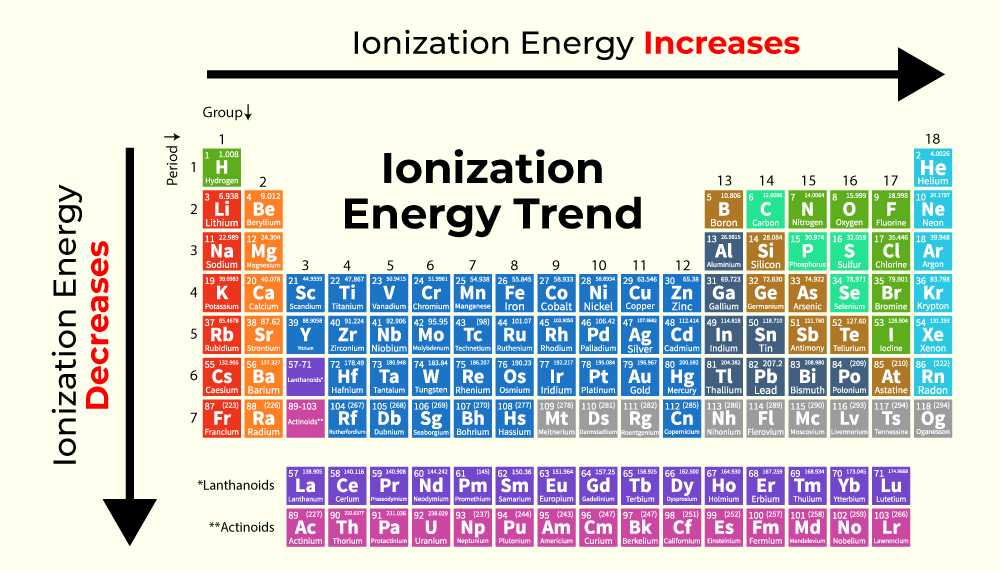
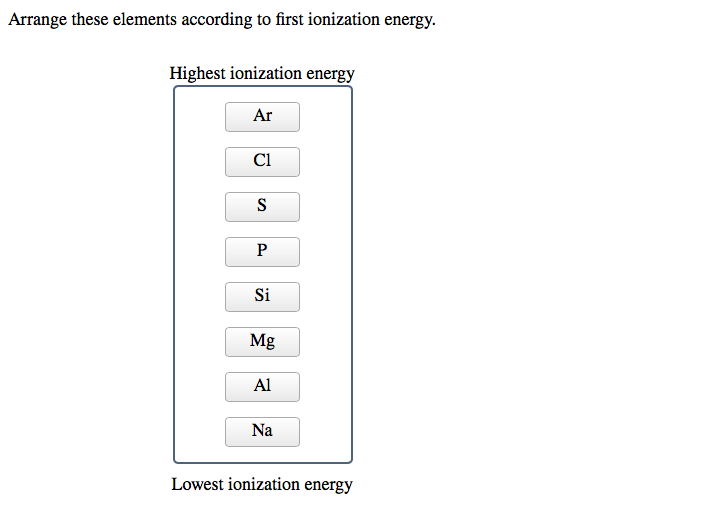
Let's Get Ranking!
Now for the fun part: ranking them! While a full periodic table ranking is extensive, let's focus on a few key players and general trends. The periodic table isn't just a random collection of elements; it's a masterpiece of organization based on recurring properties, and ionization energy is a prime example. Generally, ionization energy increases as you move across a period (from left to right) and decreases as you move down a group (from top to bottom).
Why is this the case? Across a period, the number of protons in the nucleus increases. This stronger positive charge pulls more tightly on the electrons, including the outermost ones. Even though there are more electrons, the increased nuclear pull makes it harder to remove them. So, elements on the right side of a period, like fluorine (F) or neon (Ne), are much more reluctant to give up electrons than elements on the left, like lithium (Li) or beryllium (Be).
Down a group, the story is a bit different. As you move down, atoms get larger. The outermost electrons are further away from the nucleus and are shielded by the inner electron shells. This increased distance and shielding significantly weakens the attraction between the nucleus and the outermost electron. Therefore, it takes less energy to remove that electron. This is why cesium (Cs), near the bottom of the alkali metal group, has one of the lowest ionization energies of all elements – it practically gives away its electron!
So, if we were to pick a few to rank from lowest ionization energy (easiest to remove an electron) to highest ionization energy (hardest to remove an electron), a simplified list might look something like this:
- Cesium (Cs) - Very low! Ready to ditch that electron.
- Sodium (Na) - Also quite low, but a bit more stubborn than Cesium.
- Aluminum (Al) - Somewhere in the middle, requires a bit more effort.
- Oxygen (O) - Getting harder to pull an electron away.
- Fluorine (F) - Extremely high! The electron is practically glued on.
This ranking highlights the journey from elements that readily ionize to those that strongly resist losing electrons. It's a visual representation of how their chemical personalities differ, influencing everything from their melting points to their role in biological processes. So next time you see a periodic table, remember it’s not just a chart; it’s a roadmap to understanding the fundamental forces that shape our world, one electron at a time!
