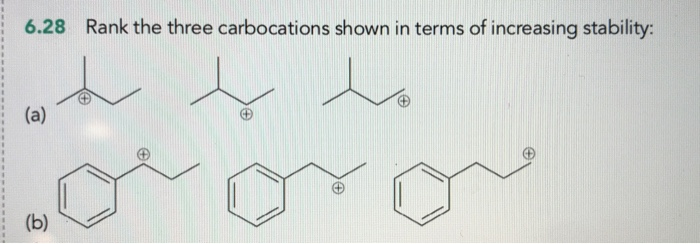
Rank The Three Carbocations Shown In Terms Of Increasing Stability:
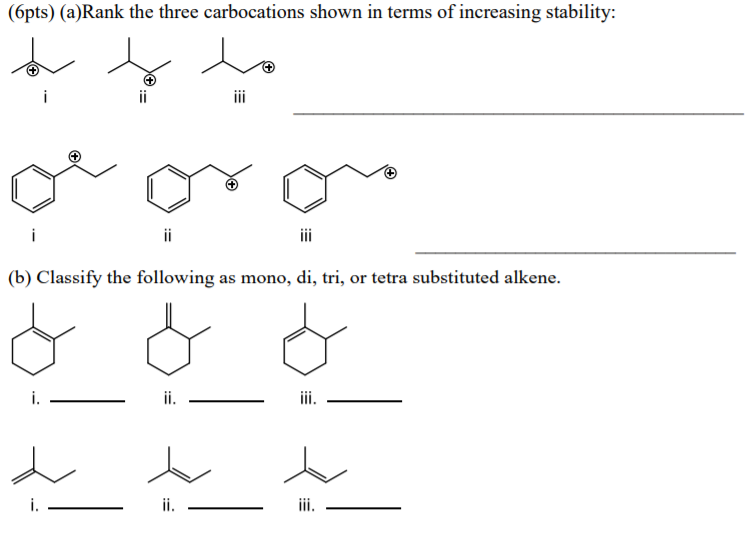
Hey there, fellow carbocation enthusiasts! Grab your coffee, settle in, because we're about to dive into something super exciting. We're talking about... carbocations! I know, I know, sounds a bit sci-fi, right? But trust me, these little dudes are the rockstars of organic chemistry. Think of them as tiny, positively charged carbon atoms just begging for some love, or, you know, electrons. And today, we're gonna rank three of 'em, from least stable to most, like a popularity contest for atoms.
So, imagine you’ve got these three characters strutting onto the scene, all with a positive charge. Who’s the shyest kid in class? Who’s the popular one everyone wants to be friends with? That’s what we’re figuring out. It's all about how well they can handle that whole "missing an electron" situation. Nature, you know, really likes things to be balanced and happy. A positive charge? Not exactly happy, if you ask me.
Think of stability like being super chill. If you’re feeling stressed, that’s not stable. If you’re on vacation, sipping a margarita, that’s peak stability. Carbocations are looking for ways to not be stressed. And in the world of chemistry, that usually means finding some electron-donating buddies.
Okay, so let's set the stage. We’ve got three carbocations. I’m gonna draw them out mentally, or, you know, you can totally picture them too. It’s like a lineup! We need to put them in order, from the least likely to survive a Tuesday morning lecture, to the ones that are basically lounging on a beach, totally unbothered.
The Contestants!
First up, we have our friend, the methyl carbocation. This little guy is just a carbon with three hydrogens attached, and a big ol' positive charge. CH3+. Super simple, super… unhappy. Why? Well, those hydrogens aren't exactly electron-donating gurus. They’re more like, "Yeah, we’re here, but don't expect much." So, this methyl carbocation is feeling pretty exposed. Like, walking into a party where you know absolutely nobody. Awkward!
Next, we’ve got the ethyl carbocation. This one’s a bit more complex. It’s got a carbon with two hydrogens and another carbon attached, and that second carbon is carrying the positive charge. CH3CH2+. Now, this ethyl group is a little more… robust. The CH3 part is a bit of a friend. It can kind of share some of its electron love. It’s not a full-on hug, more like a friendly pat on the back. But hey, that’s better than nothing, right?
And finally, we have our superstar, the tert-butyl carbocation. Oh boy, this one is living the dream. It’s a carbon with three other carbons attached to it, and the positive charge is on that central carbon. (CH3)3C+. Think of it as a party in the middle of a crowd of supportive friends. Each of those methyl groups is practically screaming, "Here, take our electrons! We've got plenty!" It’s like having your entire entourage there to back you up.
Why They’re Different (It’s All About the Friends!)
So, what’s the big deal? Why is one more stable than the other? It boils down to something called inductive effect and hyperconjugation. Don't let those fancy words scare you! Inductive effect is basically how much "electron-pushing" power a group has. Alkyl groups, like the ones made of carbon and hydrogen, are generally pretty good at donating electrons. They're like the generous folks in the neighborhood.
The more alkyl groups you can slap onto that positively charged carbon, the happier it's going to be. It’s like giving it more blankets on a cold night. Those electrons from the surrounding carbons help to delocalize the positive charge, spreading it out so it’s not so concentrated and, therefore, less intense. Less intensity means less stress, which means more stability. It’s simple math, really!
Hyperconjugation is like a little extra bonus. It’s when the electrons in adjacent C-H sigma bonds can kind of "overlap" with the empty p orbital of the carbocation. Imagine those hydrogens leaning in, whispering sweet electron-nothings to the positive center. It's a bit like a cheerleader squad for the carbocation. The more hydrogens you have on the carbons next to the positive charge, the more opportunities for this electron-sharing goodness. And guess who has the most adjacent hydrogens? Our pal, the tert-butyl carbocation!
Let’s Rank Them! (Drumroll Please!)
Alright, the moment of truth! Based on all this electron-donating, charge-spreading magic, we can finally put our carbocations in order. Remember, we’re going from least stable to most stable. Think of it as a journey from a tiny, anxious apartment to a sprawling mansion with a personal chef. Nobody wants to be in the anxious apartment, right?
The Least Stable: The Methyl Carbocation
So, at the bottom of our stability ranking, with a big, sad asterisk, is the methyl carbocation. This is our sad sack. It’s got no alkyl groups to help it out. Those hydrogens are like, "Meh." It’s basically a little ball of positive charge all by itself, with no support system. It’s like being the first person to arrive at a party and the host is still hanging up balloons. Not ideal. It’s super reactive because it’s desperately seeking electrons. It’s the equivalent of someone who’s perpetually hungry and will grab anything edible in sight. Poor thing!
Think about it – if you were feeling a bit down, would you rather be alone in a room with no one to talk to, or surrounded by your best friends who are ready to cheer you up? The methyl carbocation is that person alone in the room. It’s got no friendly electron clouds nearby to offer solace. It’s just… positive. And lonely. And that, my friends, is not a recipe for stability.
This is why, in reactions, you'll often see methyl carbocations being formed only under very specific, often high-energy, conditions, or they'll rearrange super quickly to something more stable. They're just not built for the long haul. They’re the fleeting thought, the quick decision, the impulse buy. Not built for serious contemplation or enduring relationships. They’re the rebels without a cause… or, more accurately, the carbocations without a cause-y (meaning, support).
In the Middle: The Ethyl Carbocation
Stepping up our game a bit, we have the ethyl carbocation. This one’s doing a little better. It’s got that one methyl group attached to the positively charged carbon. Remember our pat on the back? That’s the CH3 group. It's not a huge boost, but it's something. It can offer a tiny bit of electron density, a whisper of comfort. It’s like arriving at that party and there’s one person you vaguely know who smiles at you.
So, the ethyl carbocation is more stable than the methyl carbocation because that ethyl group can participate in some inductive effect. It’s a bit like having a slightly more supportive friend group. You’re not completely alone, but you’re also not the center of attention or the most popular kid. You’re somewhere in the middle, hanging in there.
It's like having a decent Wi-Fi signal versus no signal at all. It's an improvement, for sure. It’s more likely to hang around for a bit longer than our methyl friend, but it’s still not exactly kicking back with a piña colada. It’s more like, "Okay, I can handle this for a little while." It’s the student who does just enough to pass the exam without really excelling. It’s respectable, but there’s definitely room for improvement!
The Champion: The Tert-Butyl Carbocation
And now, for our reigning champion, the undisputed king of carbocation stability: the tert-butyl carbocation! This guy is the life of the party. It’s got three methyl groups all ganging up to donate electron density to that positive charge. It’s like having your entire squad there, holding you up, singing your praises. It’s the ultimate social butterfly, surrounded by fans.
This is where the inductive effect really shines. Each of those methyl groups is pushing electrons towards the positive center. And on top of that, we've got all those juicy hydrogens on the adjacent carbons ready for hyperconjugation. It's a double whammy of stability! This carbocation is so stable, it’s practically giving itself a spa treatment. It’s unfazed, unbothered, and probably wearing sunglasses indoors.
The tert-butyl carbocation is the one you’ll see formed most readily in reactions where carbocations are intermediates. It’s the rockstar that can handle the spotlight. It’s the analogy of being the CEO with a whole team of executives supporting every decision. It’s got options, it’s got backing, and it’s generally quite content. It's the reason why certain reactions proceed the way they do – because the more substituted carbocation is just so much happier and easier to form.
Putting It All Together: The Grand Finale!
So, to recap our little carbocation popularity contest, from least stable to most stable, we have:
- Methyl carbocation (CH3+): The loner, the anxious one, the one without any friends.
- Ethyl carbocation (CH3CH2+): The middle-grounder, the one with a couple of acquaintances. Better, but not amazing.
- Tert-butyl carbocation ((CH3)3C+): The superstar, the popular kid, the one with the entire entourage. Pure bliss (for a carbocation, anyway)!
It’s a pretty neat concept, right? Just by adding a few more carbon atoms and their trusty hydrogens, you can make a huge difference in how stable a charged species is. It’s all about that electron support system. Nature, in its infinite wisdom, always favors the path of least resistance, and for a carbocation, that path is paved with electron-donating groups.
So next time you’re wrestling with an organic chemistry problem and a carbocation pops up, just remember this coffee chat! Think about who’s got the most buddies, who’s got the best support network. It’s not just about memorizing, it's about understanding why. And that, my friends, is the fun part! Keep those brains buzzing and your coffees full!
