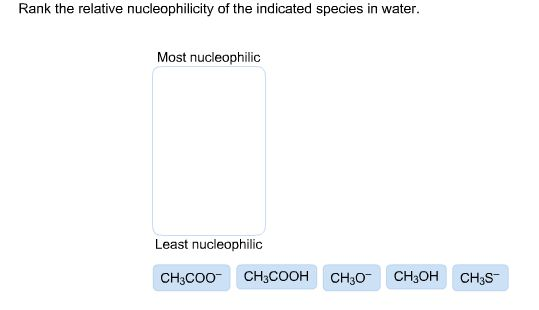
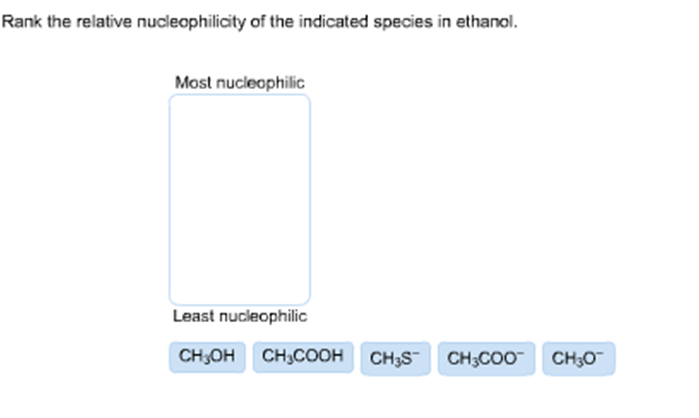
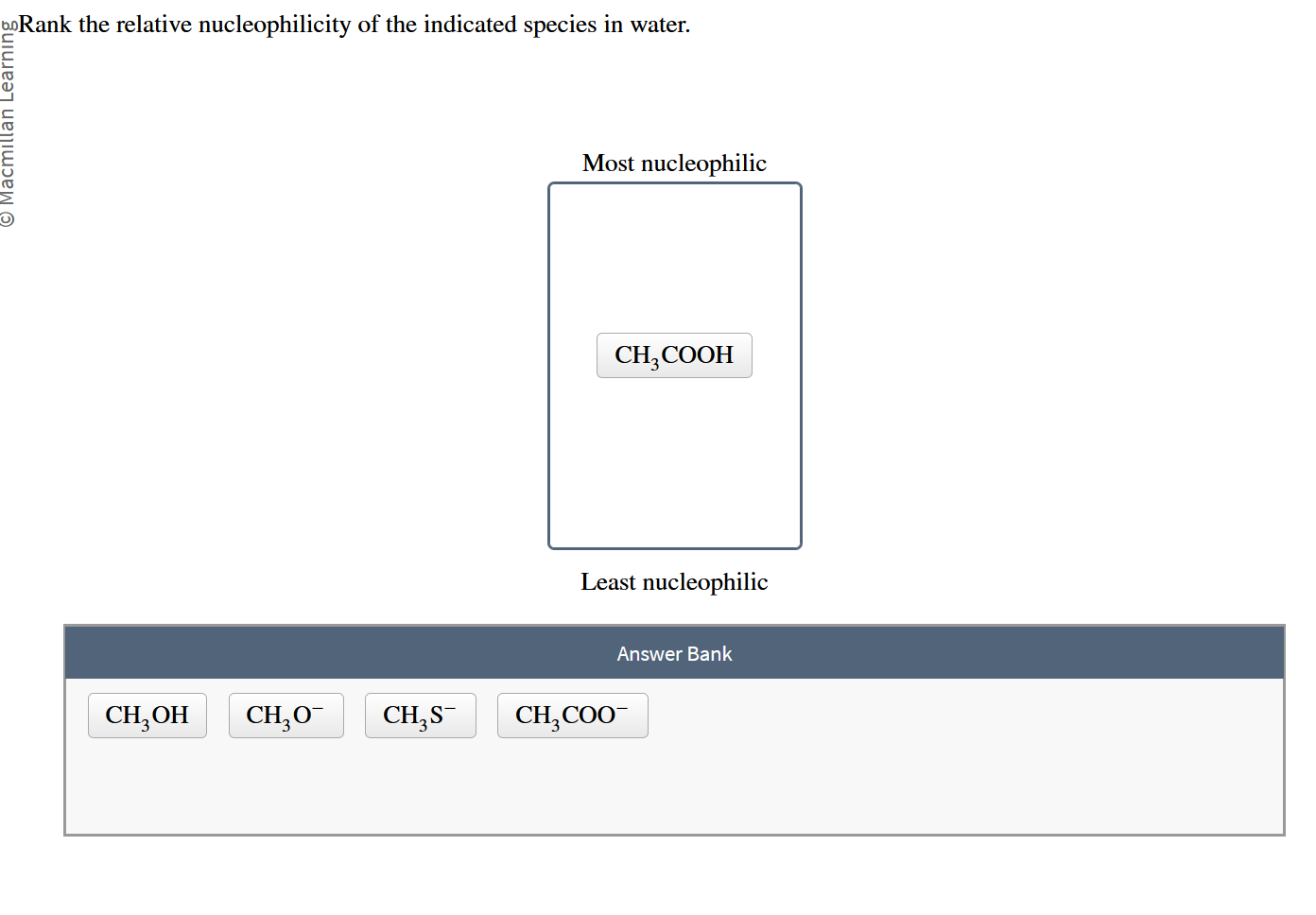
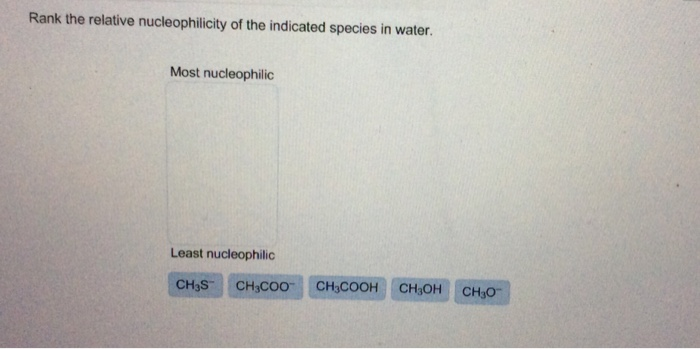
Rank The Relative Nucleophilicity Of The Indicated Species In Water.
Ever feel like you're just not attracting the right kind of attention? Well, buckle up, buttercups, because we're diving into the wild world of nucleophiles! Think of them as the friendly, outgoing personalities in the chemical world, always eager to make a connection. And where do these social butterflies hang out? Often, it's in water, the ultimate chill zone where anything can happen.
Now, imagine a party in a swimming pool. Some guests are super popular, practically mobbed by admirers. Others are a bit more reserved, waiting for someone to approach them. That's kind of what we're talking about when we rank the relative nucleophilicity of these species. It’s all about who’s the most eager to give away something valuable – in the chemical sense, this means a pair of electrons. These electrons are like little love notes, and nucleophiles are the ones writing them.
Let's meet our main players! We've got some familiar faces. First up, the ever-so-generous hydroxide ion, represented by OH-. This little guy is practically bursting with electron-giving energy. Imagine a puppy wagging its tail so hard it could knock over a lamp – that’s OH-! It’s a powerhouse of nucleophilicity, always ready to jump into action and form a new bond. It’s like the life of the chemical party, always the first to offer a dance, a compliment, or, you know, electrons.
Then we have water itself, H2O. Now, water is usually seen as a neutral bystander, a good solvent for all sorts of shenanigans. But even water has a little spark! It’s not as gung-ho as OH-, but it’s definitely willing to share its electron love when the opportunity arises. Think of water as that polite friend who will always offer you a sip of their drink – not as exuberant as the puppy, but still a good sport.
Next, we bump into something called hydronium ion, or H3O+. This one’s a bit different. It’s got a positive charge, which makes it a bit… well, less inclined to give away electrons. It’s already feeling a bit needy, you see. Imagine someone who’s just lost their wallet; they're not exactly in a generous mood, are they? They're more likely to be looking for electrons rather than giving them away. So, compared to our friendly OH-, H3O+ is much less of a social butterfly in the electron-sharing department.
Now, let's put them all together in our water party. Who’s going to be the most popular? Who's going to get all the attention? Well, the hydroxide ion (OH-) is definitely the superstar. It’s practically overflowing with electrons, making it the most attractive option for any electron-craving molecule. It’s the one everyone wants to be around, the one initiating the most connections.
Following closely behind is good old water (H2O). It's still a good contender, willing to participate in the electron exchange, just not with the same frantic enthusiasm as OH-. It’s like the reliable friend who’s always there, happy to join in the fun but not necessarily the one organizing the whole shindig.
And then we have the hydronium ion (H3O+). It’s kind of the wallflower at this particular party. It’s got this positive vibe going on, which means it’s more interested in receiving electron affection than giving it out. It's the one you might have to go up to and initiate a conversation with, and even then, it might be a bit hesitant to share its inner secrets (or electrons).
So, when we talk about ranking nucleophilicity in water, we're essentially ordering them by how good they are at sharing those precious electrons. It’s a simple concept, but it explains so much about how different molecules interact and form new friendships. It’s like a popularity contest, but instead of votes, it’s based on electron generosity!
It’s a little surprising, isn't it? That something as seemingly simple as an ion or a molecule can have such a distinct personality when it comes to forming connections. The hydroxide ion, with its negative charge, is like a magnet for positive attention, eagerly offering up its electron wealth. Water, though neutral, has a subtle charm and is always ready to play along. And the hydronium ion, with its positive charge, is on the receiving end, a bit pickier about who it lets get close.
It’s a beautiful dance of attraction and repulsion, a constant push and pull that drives chemical reactions. And to think, it all happens in the everyday medium of water! So next time you’re enjoying a glass of water, remember the invisible world of nucleophiles, the electron-givers, and their fascinating social lives. It’s a reminder that even in the seemingly mundane, there's a whole lot of exciting chemistry going on, just waiting to be appreciated!
