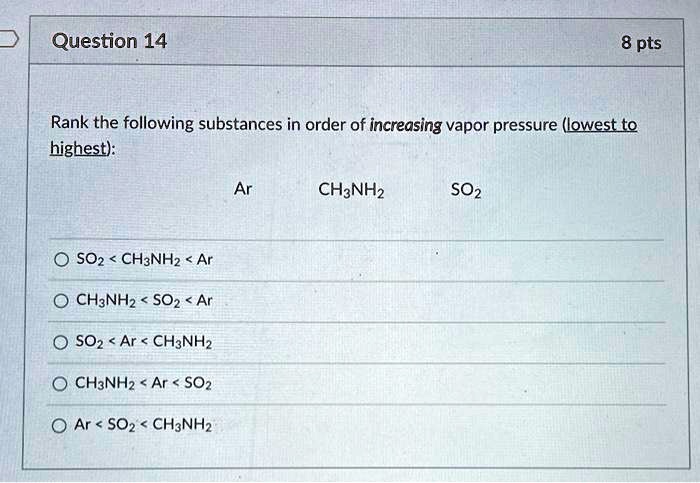
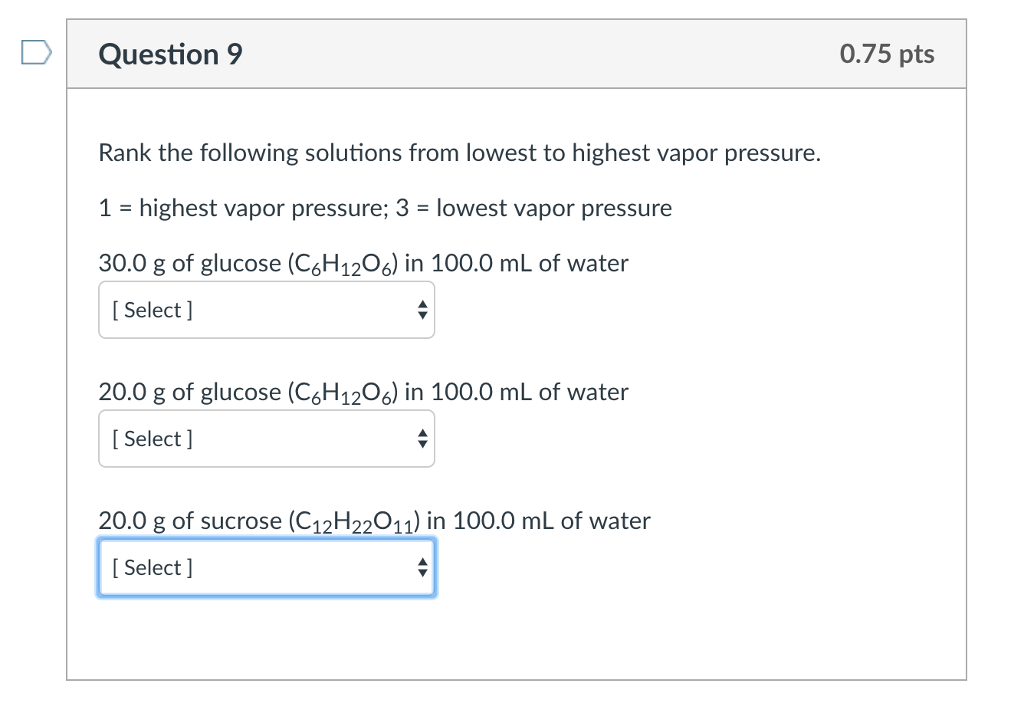
Rank The Following Solutions From Lowest To Highest Vapor Pressure.

Hey there, fellow wanderers of the everyday! Ever find yourself staring at a steaming mug of coffee, or maybe just the subtle sheen on a perfectly chilled glass of lemonade, and wonder about the invisible dance happening all around us? We're talking about something so fundamental, so everywhere, yet often overlooked: vapor pressure. Now, before your eyes glaze over and you mentally check out for a spa day, let me assure you, this isn't some dry, lab-coat-required kind of topic. Think of it as unlocking a little secret of how the world works, a subtle superpower that explains everything from why your clothes dry faster on a breezy day to why that perfume you love lingers so beautifully (or not so beautifully, depending on the scent!).
We're going to dive into a fun little ranking exercise. We've got a lineup of everyday heroes, substances we encounter constantly, and we're going to sort them out based on their tendency to, well, evaporate. It’s like a popularity contest, but for molecules on the move!
The Evaporation Equation: It's All About the Vibe
So, what exactly is vapor pressure? In the simplest terms, it's the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. Sounds fancy, right? But let's break it down. Imagine a sealed bottle of water. Some water molecules are chilling in the liquid, while others, with a bit more energy, have broken free and are zipping around as vapor above the water. When the rate of evaporation equals the rate of condensation, you've got equilibrium. The pressure those vapor molecules are exerting? That's your vapor pressure. Higher vapor pressure means more molecules are eager to escape into the gaseous state. Think of it as their "get up and go" attitude.
What influences this "get up and go"? Two main things: temperature and the intermolecular forces holding the molecules together. The hotter it gets, the more energy those molecules have, and the more likely they are to make a break for it. It's like a party – the more energetic the guests, the more likely they are to spill out onto the dance floor (or, in this case, the air!). And those intermolecular forces? If they're strong, like a group hug, the molecules are less likely to separate. If they're weak, it's easier for them to go their own way.
Our Lineup: From Chilled to Thriving
Alright, let's meet our contestants! We've got a diverse bunch, representing different aspects of our daily lives:
- Water (H₂O): The ultimate hydration hero, found everywhere from your morning shower to that refreshing glass you sip on.
- Ethanol (C₂H₅OH): The sophisticated sipper, present in your favorite glass of wine, that celebratory champagne, or even the hand sanitizer you've become so familiar with.
- Acetone (C₃H₆O): The quick-drying star, the go-to in nail polish remover, often found lurking in art studios and craft rooms.
- Hexane (C₆H₁₄): A less glamorous but essential character, often found in solvents and fuels, a workhorse behind the scenes.
- Glycerol (C₃H₈O₃): The humectant heavyweight, a moisturizer's best friend, known for its sticky-sweet, syrupy nature.
Now, the mission: rank these from the lowest vapor pressure to the highest vapor pressure at a typical room temperature (let's say around 25°C or 77°F). This means we're looking for the substances that are most reluctant to become vapor, all the way to the ones that are practically begging for a vacation in the air.

The Ranking Reveal: From Slow Movers to Swift Escapers
Let's get down to the nitty-gritty and see where each of our contenders stands. Prepare for some eye-opening revelations!
#1: Glycerol (C₃H₈O₃) - The Steadfast One
At the very bottom of our vapor pressure ladder, we have glycerol. This is the molecule that’s in no hurry whatsoever to leave the liquid phase. Why? Because glycerol is a master of hydrogen bonding. Think of it as having multiple arms, each one reaching out to its neighbors and giving them a really, really tight hug. These strong intermolecular forces make it incredibly difficult for glycerol molecules to break free and become a gas. It’s viscous, it’s syrupy, and it sticks around. This is why it's such a fantastic ingredient in moisturizers – it creates a protective barrier and holds onto moisture, keeping your skin supple. It’s the reliable friend who’s always there, never causing a fuss, and definitely not prone to spontaneous combustion or disappearing acts.
Fun Fact: Glycerol is also used in some types of e-cigarettes as a base for the liquid. Its low volatility means it produces a thicker, more visible vapor when heated, which is part of the vaping experience!

#2: Water (H₂O) - The Ever-Present Player
Next up, we have our ubiquitous friend, water. Water also engages in hydrogen bonding, though not quite as extensively as glycerol. Those water molecules like to hold hands, but their grip isn't as ironclad. This allows water to evaporate at a reasonable rate, which is crucial for so many natural processes, like cloud formation and the water cycle. It's why puddles disappear after a rain shower, and why washing your hands feels refreshing. The vapor pressure of water is what makes things feel humid when it's high, and dry when it's low. It’s the Goldilocks of molecules – just right for life as we know it.
Practical Tip: On a humid day, when water’s vapor pressure is higher, drying clothes indoors can make your living space feel even muggier. Hanging them outside, especially on a breezy day, will speed up evaporation significantly!
#3: Ethanol (C₂H₅OH) - The Spirited Contender
Moving up the ladder, we encounter ethanol. Ethanol molecules also participate in hydrogen bonding, but they have a shorter, less polar "tail" than water. This means the forces holding them together are a bit weaker. Think of it as a slightly less enthusiastic handshake compared to water. Consequently, ethanol has a higher vapor pressure than water. This is why that glass of wine or that shot of spirits “breathes” and releases its aroma – the ethanol is readily becoming vapor. It’s also why rubbing alcohol (which is mostly ethanol) evaporates so quickly from your skin, leaving you with that cool sensation.
Cultural Nod: The pleasing aroma of wine, often described with notes of cherry, oak, or even leather, is largely due to the volatile compounds, including ethanol, that are released into the air. It’s a testament to ethanol’s willingness to become airborne!
#4: Hexane (C₆H₁₄) - The Practical Workhorse
Now we get to hexane, a molecule that’s a bit different. Unlike water, ethanol, and glycerol, hexane is a nonpolar molecule. This means its molecules primarily interact through weaker forces called London dispersion forces. Imagine them as very light, fleeting hand-fives rather than solid handshakes. Because these forces are much weaker, hexane molecules are far more eager to escape into the vapor phase. This gives hexane a significantly higher vapor pressure than the hydrogen-bonding compounds we’ve discussed. It’s this property that makes it a useful solvent for things like oils and greases, as it can easily dissolve and carry them away in its vapor state.
Did You Know?: Hexane is a component in many petroleum products. Its tendency to evaporate readily is why gasoline fumes can be quite potent and why proper ventilation is so important when dealing with fuels.
#5: Acetone (C₃H₆O) - The Speedy Evaporator
And finally, reigning supreme in our vapor pressure contest, we have acetone! Acetone is a polar molecule due to the oxygen atom, but it's quite small and has weaker intermolecular forces (dipole-dipole interactions and London dispersion forces) compared to water or ethanol. Its structure allows its molecules to move around and escape into the gas phase with remarkable ease. This is why nail polish remover, typically acetone-based, dries in a flash. It's the ultimate "here today, gone tomorrow" substance on our list. It’s the pop star of evaporation, always eager to hit the stage – or the air!
Life Hack: Need to quickly clean a sticky residue from a label? Acetone can be your best friend, but be sure to use it in a well-ventilated area and away from open flames, as it's highly flammable!
The Big Picture: More Than Just Lab Notes
So, there you have it! Our journey from the steadfast glycerol to the zippy acetone, all ranked by their vapor pressure. It’s a neat way to understand how these different substances behave, and it has practical implications in so many areas of our lives. From the subtle scents that drift through your kitchen to the way your laundry dries on the line, vapor pressure is silently at play.
Think about it: why does a delicate perfume evaporate and dissipate faster than a heavy, oily essential oil? It’s their differing vapor pressures at work. Why does a hot cup of tea cool down faster than a cold one? The hotter liquid has more energy, leading to increased evaporation and thus faster cooling. It’s all connected, and a little understanding of molecular behavior can add a fascinating layer to your everyday observations.
A Little Reflection: The Dance of Presence and Absence
As I sip my morning coffee, the steam rising from the mug is a visible reminder of water molecules transitioning from liquid to gas. It's a moment of quiet observation, a subtle acknowledgment of the invisible forces that shape our world. We often focus on what's tangible, what we can see and touch, but there's a whole universe of activity happening in the unseen realms – the constant dance of molecules. Understanding concepts like vapor pressure, even in a lighthearted way, reminds us that the world is a dynamic and ever-changing place. It’s a gentle nudge to appreciate the subtle complexities that make our daily lives, and the world around us, so wonderfully intricate. So next time you notice something evaporating, from a dewdrop on a leaf to the last drop of your favorite cleaning spray, take a moment. You’re witnessing a little bit of molecular magic, a testament to the continuous, quiet hum of existence.
