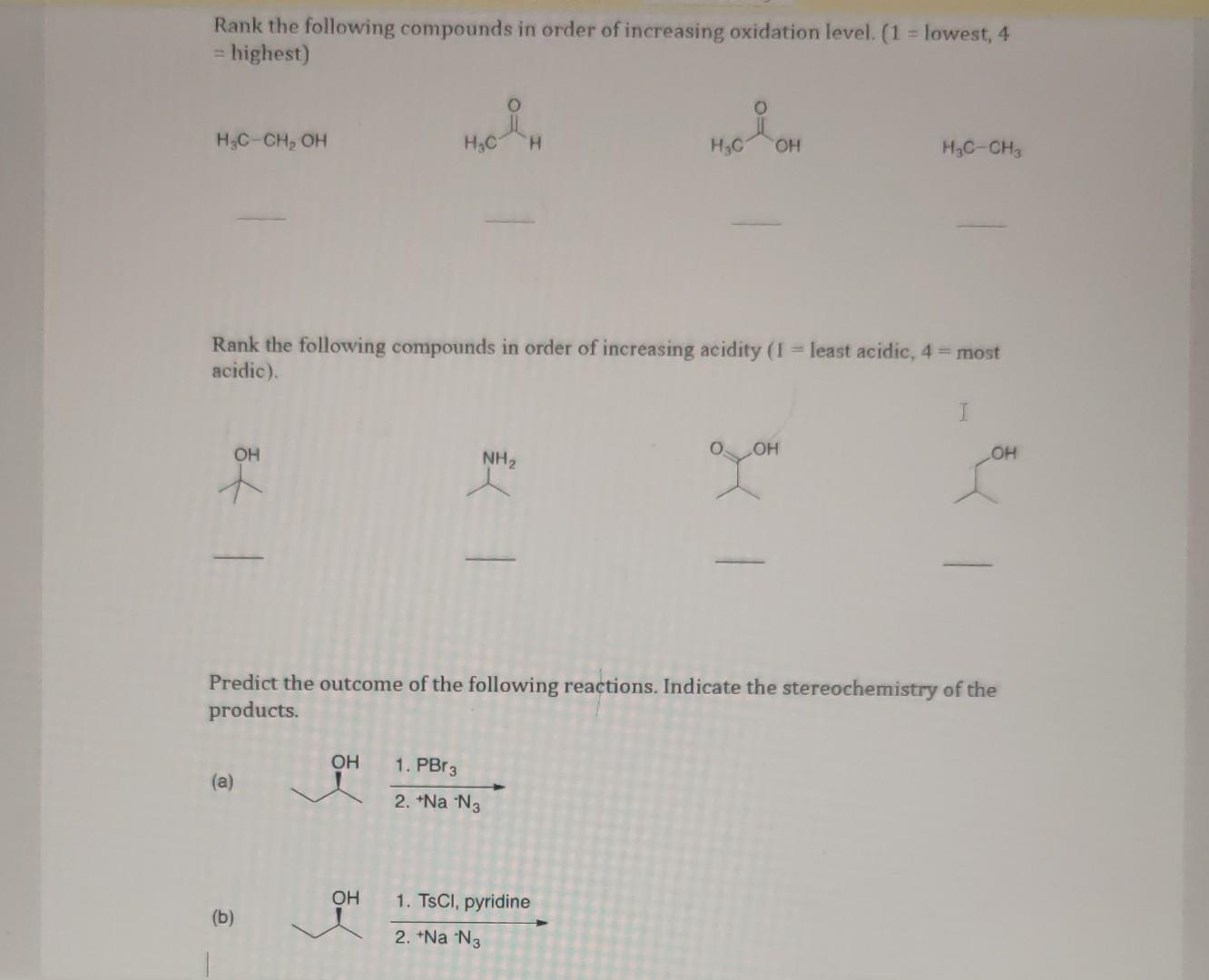
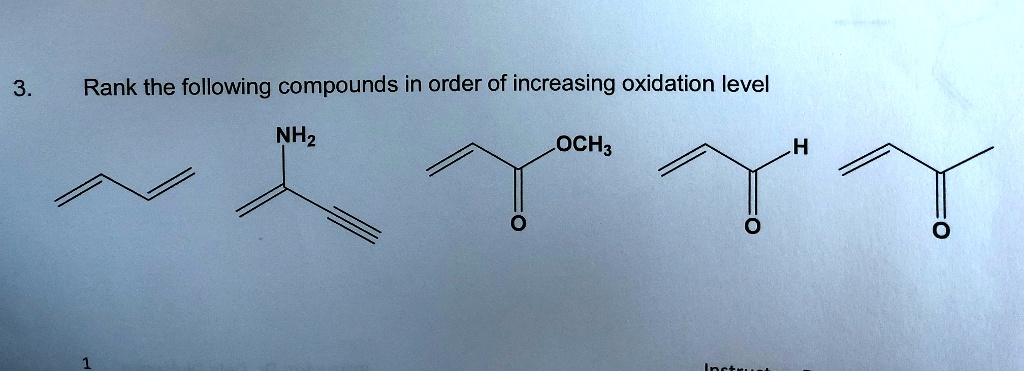
Rank The Following Compounds In Order Of Increasing Oxidation State
So, I was having one of those days, you know? The kind where you stare into the abyss of your coffee cup and wonder if your life choices have led you to this very moment of questioning the fundamental nature of… well, everything. And today, that "everything" involved a rather peculiar list of chemical compounds. My brain, usually buzzing with the usual delightful chaos of everyday life, suddenly got a bit… nerdy.
I was staring at a spreadsheet, trying to make sense of some data, and a random thought popped into my head: what if I could rank these things? Not by their color, or their smell (thank goodness for that!), but by something more… intrinsic. Something that tells me a little bit about their inner workings. And then it hit me, like a rogue electron jumping a shell: oxidation states!
Now, before you click away thinking this is going to be some dry, textbook lecture, hang in there! I promise, it’s not. Think of oxidation states like a little scorekeeping system for atoms. It’s how we figure out who’s been a good electron citizen and who’s been a bit of a thief. And when we start ranking compounds based on these scores, things get surprisingly interesting. It’s like peeking behind the curtain of chemical reactions, seeing who’s holding what, and why they might be doing it.
The Great Oxidation State Shuffle: Let's Rank Some Stuff!
Alright, so the compounds we’re going to be playing with today are:
- Water (H₂O)
- Methane (CH₄)
- Ammonia (NH₃)
- Carbon Dioxide (CO₂)
- Sulfuric Acid (H₂SO₄)
My mission, should I choose to accept it (and my coffee clearly convinced me I should), is to arrange these guys in order of increasing oxidation state. What does that even mean? Well, in simple terms, it’s a way to assign hypothetical charges to atoms within a compound. It’s not a real charge, mind you, but it helps us understand how electrons are shared or transferred during chemical bonding.
Think of it like this: imagine each atom is at a party. Some atoms are super generous and tend to give away their electrons (they get a negative oxidation state, like they’re owing something). Others are a bit greedy and tend to snatch electrons from their neighbors (they get a positive oxidation state, like they’re rich in electron-stealing power). The oxidation state is basically their final score after all the electron mingling at the party.
Getting Our Hands Dirty (Metaphorically, Of Course)
To figure out the oxidation state of an atom in a compound, we rely on a set of rules. These are like the unwritten laws of the atomic party. For the most part, these rules are pretty consistent:
- Oxygen usually has an oxidation state of -2. (Unless it's in a peroxide, then it's -1, or with fluorine, then it can be positive. Chemistry, am I right? Always with the exceptions!)
- Hydrogen is usually +1 when bonded to non-metals. (When it's with metals, it can be -1. See? More exceptions!)
- The oxidation state of an element in its elemental form (like O₂ or H₂) is always 0.
- The sum of the oxidation states of all atoms in a neutral compound must equal zero.
- The sum of the oxidation states of all atoms in an ion equals the charge of the ion.
These rules are our trusty compass for navigating the world of oxidation states. They’re not always perfect, but they give us a really good starting point for understanding the electron distribution in molecules.
Let’s Break Down Each Compound
Now, let’s apply these rules to our list. This is where the real fun begins, folks!
1. Methane (CH₄)
Here, we have carbon (C) and four hydrogens (H). Hydrogen, when bonded to a non-metal like carbon, usually gets a +1 oxidation state. Since there are four hydrogens, that’s a total of +4 from them.
Remember, the whole molecule is neutral, so the sum of the oxidation states must be zero. If the hydrogens contribute +4, then the carbon must be -4 to balance it out. So, in methane, carbon is chilling with a -4 oxidation state. It's the ultimate electron donor in this scenario, being super generous with its electrons to those greedy hydrogens!
2. Water (H₂O)
Ah, water! The elixir of life, the basis of all hydration-related conversations. In water, we have two hydrogens (H) and one oxygen (O). Hydrogen, as we know, is usually +1. So, the two hydrogens give us a total of +2.
Oxygen, on the other hand, is usually -2. Does that balance out? Yep! +2 from the hydrogens and -2 from the oxygen makes a grand total of 0. So, in water, hydrogen is +1 and oxygen is -2. Oxygen is definitely the electron hog here, pulling those electrons towards itself!
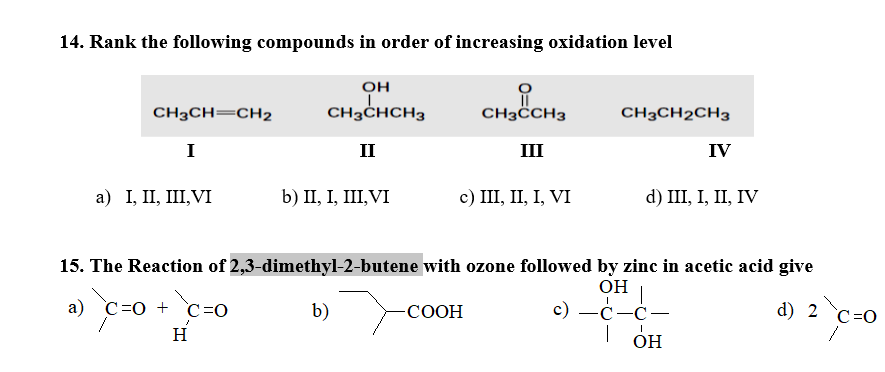
3. Ammonia (NH₃)
Moving on to ammonia! This one has nitrogen (N) and three hydrogens (H). Again, hydrogen is playing its usual +1 role, contributing a total of +3.
To keep the molecule neutral, nitrogen has to be -3. So, just like in methane, we have a highly negative oxidation state on one of the atoms. Here, nitrogen is acting like the super-generous one, giving away its electron density.
4. Carbon Dioxide (CO₂)
Carbon dioxide. The stuff we exhale, the stuff that keeps our planet warm (sometimes a little too warm, ahem). This molecule has one carbon (C) and two oxygens (O). Oxygen, our usual suspect, is -2. With two oxygens, that’s a total of -4.
To make the whole thing add up to zero, the carbon has to be +4. Wowza! Talk about a flip in personality from methane. In carbon dioxide, carbon is the electron thief, aggressively pulling electrons from both oxygens. This is a prime example of how the same atom can have vastly different oxidation states depending on what it's bonded to.
5. Sulfuric Acid (H₂SO₄)
Alright, the grand finale: sulfuric acid. This is a bit more complex, with hydrogen (H), sulfur (S), and oxygen (O). We’ve got two hydrogens, each at +1, giving us +2. We have four oxygens, each at -2, giving us -8.
So, we have +2 from the hydrogens and -8 from the oxygens, which totals -6. To make the whole molecule neutral, the sulfur must be +6. That's the highest positive oxidation state we've seen so far! Sulfur here is truly playing the role of the electron-grabbing champion.
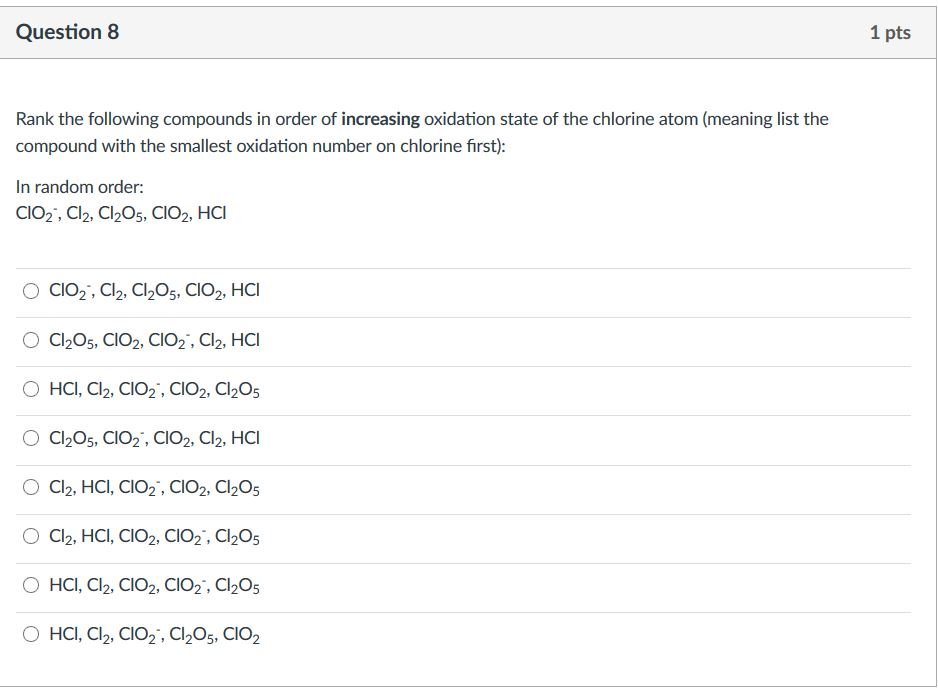
The Grand Ranking Reveal!
Okay, are you ready for the moment of truth? We’ve done the detective work, we’ve applied the rules, and now it’s time to put these compounds in order from the lowest (most negative) oxidation state to the highest (most positive) in terms of the *most negative atom within the compound. Why the most negative atom? Because that's often where the action is, where the electron-stealing or electron-donating is most pronounced and defines the compound’s character in this context.
Drumroll, please…
-
Methane (CH₄)
Here, carbon has an oxidation state of -4. This is our lowest, most electron-rich atom we've encountered. It’s the king of giving electrons away in this group!
-
Ammonia (NH₃)
In ammonia, nitrogen has an oxidation state of -3. It’s not quite as electron-rich as the carbon in methane, but it’s still strongly donating electrons.
-
Water (H₂O)
Water has our oxygen at -2. While oxygen is a strong electron taker, compared to the negative charges on carbon and nitrogen, it’s a slightly less extreme negative in this comparison.
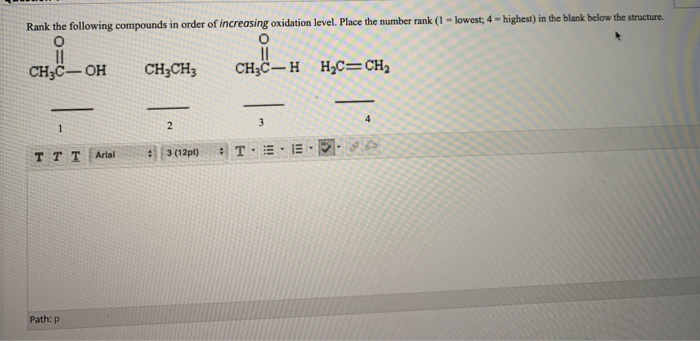
 SOLVED: Rank the following compounds in order of increasing oxidation
SOLVED: Rank the following compounds in order of increasing oxidation -
Carbon Dioxide (CO₂)
Now we're switching gears! In CO₂, carbon has an oxidation state of +4. This is a big leap into positive territory. Carbon is now acting as a significant electron *acceptor.
-
Sulfuric Acid (H₂SO₄)
And finally, sulfuric acid! The sulfur here is at a whopping +6. This is our most electron-deficient atom, having effectively 'lost' the most electrons in this hypothetical electron exchange. It’s the ultimate electron hoarder!
Why Does This Even Matter? (Besides Satisfying My Curiosity)
So, we’ve ranked them. Neat, right? But beyond being a fun mental exercise, understanding oxidation states is super important in chemistry. It helps us predict how reactions will occur. For example, a compound with a very low oxidation state is likely to be a good reducing agent (it can donate electrons easily), while a compound with a high oxidation state is likely to be a good oxidizing agent (it can accept electrons easily).
Think of redox reactions (short for reduction-oxidation reactions) as a chemical tug-of-war for electrons. The oxidation states tell us who's on which side and how strong they are. It's fundamental to understanding everything from how batteries work to how our bodies metabolize food!
It's also a fantastic way to notice patterns. See how carbon can be both -4 and +4? That's a clue that carbon is incredibly versatile and can form a wide range of bonds, which is why organic chemistry (the chemistry of carbon compounds) is so vast and fascinating. And that super high +6 for sulfur in sulfuric acid? That hints at its powerful oxidizing abilities.
So, next time you're staring into your coffee, or a puddle, or really anything containing chemicals, you can remember the humble oxidation state. It’s a little number that tells a big story about the electron dynamics at play. And who knows, it might just make your day a little more interesting. Or at least, give you something to ponder while you wait for your toast to pop. Happy oxidizing, my friends!
