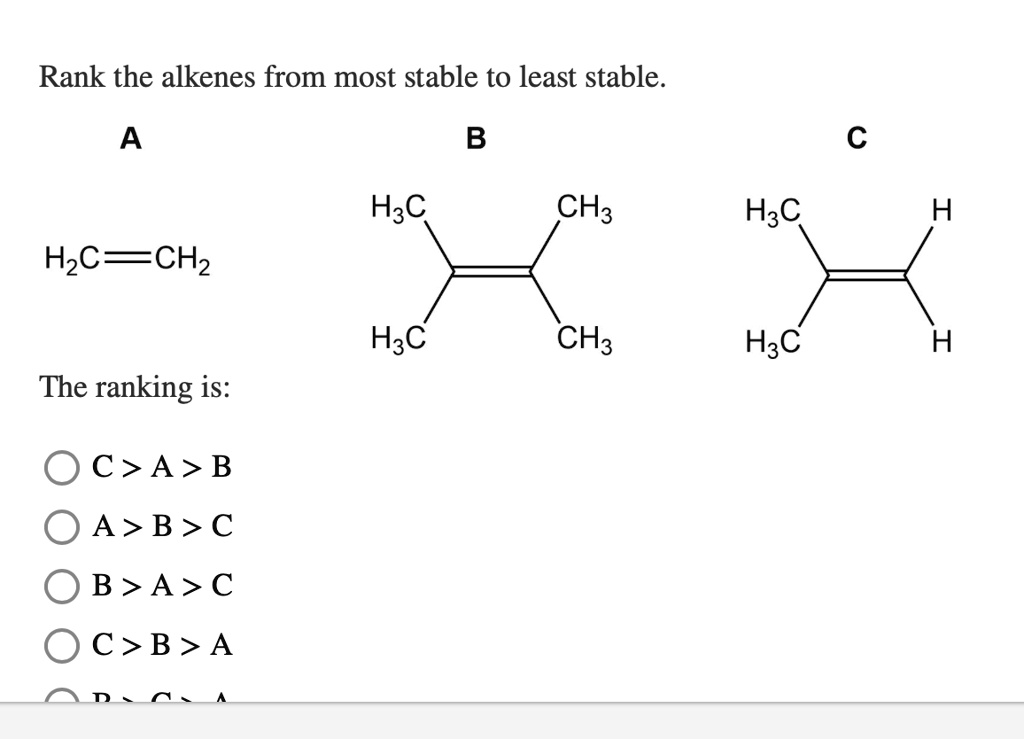
Rank The Alkenes Below From Most Stable To Least Stable.
Alright everyone, buckle up your chemical roller skates because we're about to dive into the wild and wonderful world of alkenes! Think of these guys like the party animals of the molecule world. They’ve got that super-duper double bond, which makes them a little bit more… excitable than their less flashy, single-bonded cousins, the alkanes.
Now, sometimes, life throws us a curveball. We’ve got a bunch of these alkene party animals lined up, and our mission, should we choose to accept it (and we totally do, because it's FUN!), is to figure out who’s the most chill and who’s the most ready to spontaneously combust into a burst of pure, unadulterated energy. It’s all about stability, folks!
Imagine you’re at a massive party. Some guests are just vibing, happily chatting in a corner. Others are practically bouncing off the walls, ready for the next dance-off. That's kind of what we're doing here, but with molecules and their double bonds. The more stable an alkene, the more it’s like that person who’s content with a good conversation and a comfortable sofa.
The less stable ones? They’re the ones crowd-surfing, looking for the next adventure. It’s a spectrum, and we’re here to sort it out, from the ultimate chillers to the ultimate thrill-seekers. Get ready for some serious molecular matchmaking!
So, we've got our contenders. Let's call them Contender A, Contender B, Contender C, and Contender D. Each of these represents a different alkene, with its own unique arrangement of atoms and that all-important double bond. Our quest today is to put them in order, from the most laid-back and stable to the most energetic and, well, less stable.
Think of it like this: you're ranking your favorite snacks. Some are perfectly satisfying, like a warm cookie. Others are a sugar rush waiting to happen, like that neon-colored candy. We’re doing the same for our alkenes, but with much more scientific flair and way less dental worry.
Our ranking is based on a super cool, but thankfully simple, concept. It’s all about how many other carbon friends are hugging that double bond. The more friends, the more they can share their energy and help keep things steady. It’s like a group hug for the double bond, making it feel all warm and fuzzy and, you guessed it, stable!
So, a double bond that has lots of carbon buddies attached to it is going to be happier and more content. It’s got a built-in support system, and that makes all the difference in the world. This is the secret sauce, the magic ingredient to our ranking!
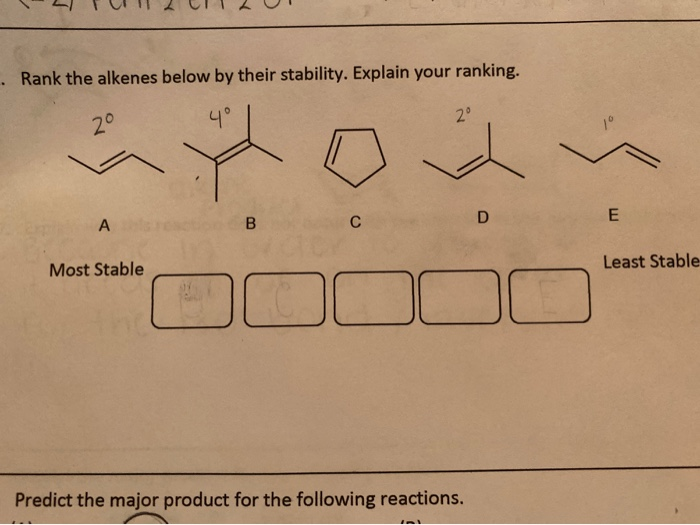
Let's introduce our contestants properly. We have:
Contender A: The King of Chill
This alkene is the absolute life of the party, but in the most responsible way. It’s got its double bond surrounded by a veritable posse of carbon friends. We're talking two carbon atoms directly attached to each side of the double bond. This is the most substituted alkene you can get!
Imagine this guy is at the party, and he’s got two best friends on one side and two best friends on the other, all having a blast. They're all sharing their good vibes, and the double bond is just basking in the glow. It's like a perfectly balanced symphony of awesome!
Because it has so many carbon connections, it’s super stable. It’s not looking to react with anything in a hurry because, frankly, it's already having too good a time being itself. This is our ultimate champion of stability. Give it a round of applause!
Contender B: The Energetic Enthusiast
This alkene is right there with the king, but with just a tiny bit less company. It's got its double bond with one carbon atom attached to one side and two carbon atoms attached to the other. Still a fantastic amount of support!
Think of this one as having a solid group of three friends around the double bond. They’re still having an amazing time, sharing jokes and good energy. It’s a little less of a crowd than Contender A, but still a very, very happy gathering.
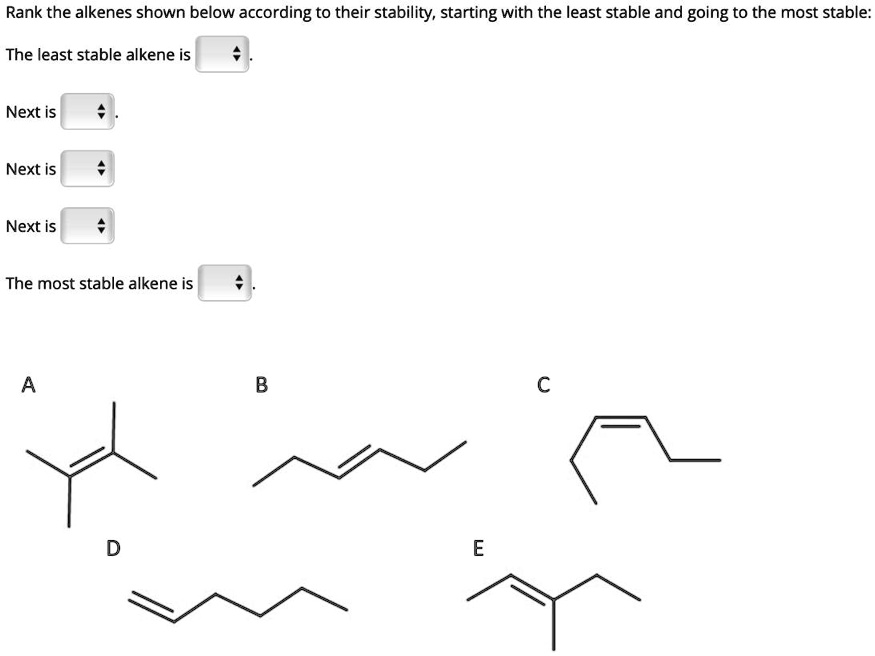
This alkene is also incredibly stable, just a smidge less so than our absolute top dog. It’s still got plenty of friends to lean on, so it’s not itching for a wild adventure. It’s more like, “Yeah, let’s have fun, but let’s keep it relatively chill.”
Contender C: The Social Butterfly
Now we’re getting to the ones who are a little more ready to mingle. This alkene has its double bond connected to one carbon atom on one side and one carbon atom on the other side. It's got two carbon friends in total.
Picture this: the double bond has two friends who are really close by, chatting away. It’s a nice, cozy little group, but it’s not quite the mega-party of our previous contenders. There’s a bit more space around the double bond, making it slightly more… accessible.
This alkene is still pretty happy, don't get me wrong. It’s got some friends to share the spotlight with. But compared to the others, it’s got a bit more of an adventurous spirit. It’s like, “Okay, we’re having fun, but what else is out there?”
Contender D: The Lone Ranger
And here we have our final contestant. This alkene has its double bond connected to only one carbon atom on one side, and the other side is connected to something else, usually a hydrogen atom. It has just one carbon friend directly attached to the double bond.
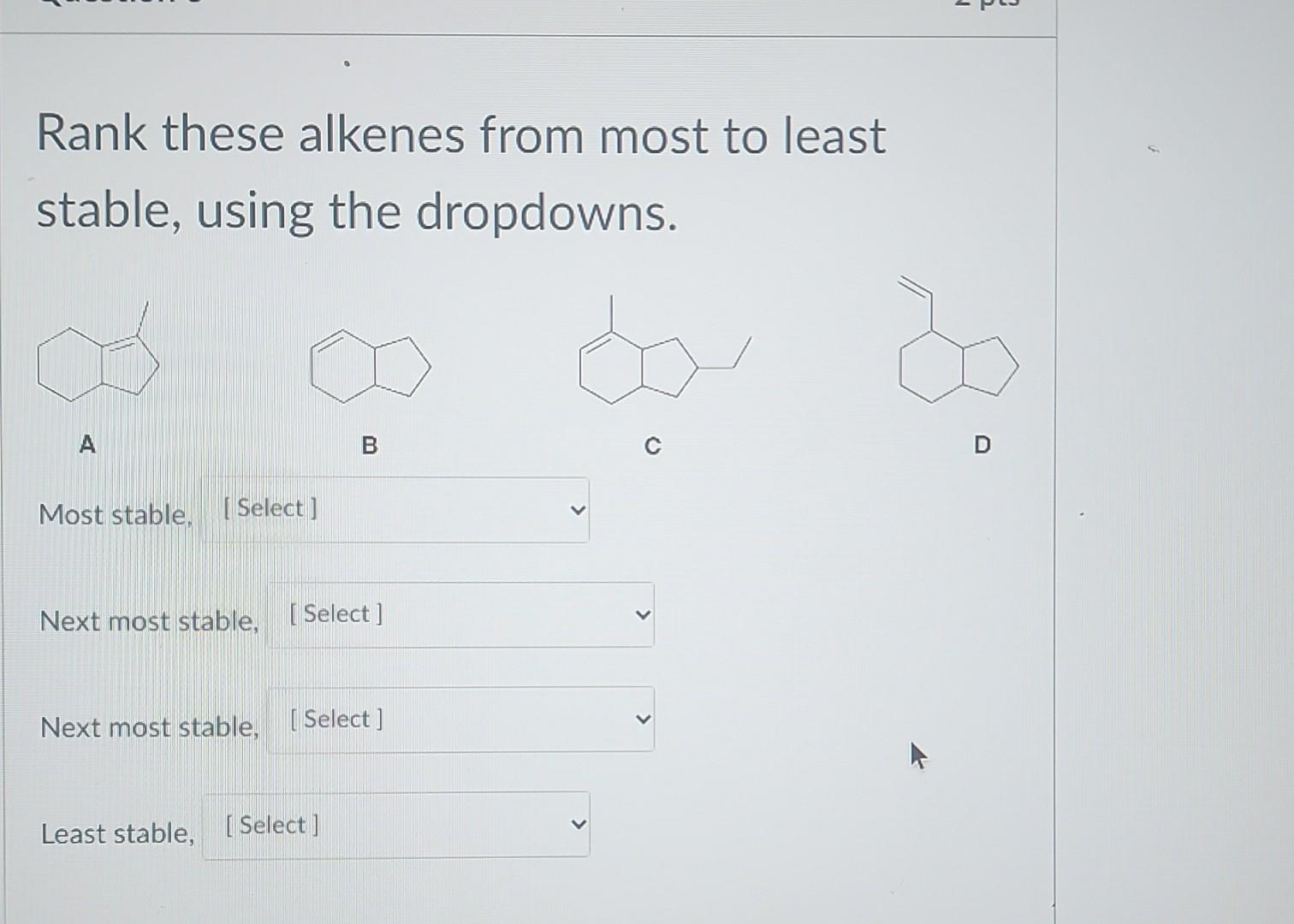
Imagine this double bond has only one buddy to hang out with. The rest of the space around it is a bit more… open. It's like that one person at the party who's mostly by themselves, looking around.
Because it has fewer carbon attachments, it’s the least stable of our group. That double bond is a bit more exposed and ready to get into some reactions. It's the alkene that's most likely to say, "I’m bored, let's do something crazy!" This is our thrill-seeker, our daredevil.
So, let’s put it all together! We’re ranking them from the most stable (the ultimate chillers) to the least stable (the ready-for-anything adventurers).
Our ranking goes like this:
Most Stable: Contender A (The King of Chill - four carbon attachments to the double bond! It’s like a five-star resort for double bonds!)
Next Up: Contender B (The Energetic Enthusiast - three carbon attachments! Still a fantastic party, just a slightly cozier vibe.)
[SOLVED] Rank the alkenes below from most stable to least stable (mostGetting Lively: Contender C (The Social Butterfly - two carbon attachments! It's mingling and enjoying the scene, but open to more.)
Least Stable: Contender D (The Lone Ranger - one carbon attachment! This double bond is ready for its close-up and a bit of action!)
Isn’t that neat? It’s all about those carbon buddies giving the double bond a sense of belonging and support. The more buddies, the more stable the alkene. Simple as that!
So next time you’re looking at some alkenes, you can impress your friends with your newfound ability to rank their stability. You'll be the molecular party guru, knowing exactly who’s ready to chill and who’s ready to… well, do something chemically exciting!
It’s a beautiful science, isn't it? Understanding these tiny, energetic molecules and how they relate to each other. Each alkene has its own personality, its own level of exuberance, and by understanding their structure, we can predict their behavior.
And that, my friends, is how you rank alkenes from most stable to least stable. It’s a journey from ultimate contentedness to eager anticipation, all thanks to the power of those friendly carbon attachments. Now go forth and spread the alkene love!

![[SOLVED] Rank the alkenes below from most stable to least stable (most](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f0784facd25_47166f0784f529fe.jpg)