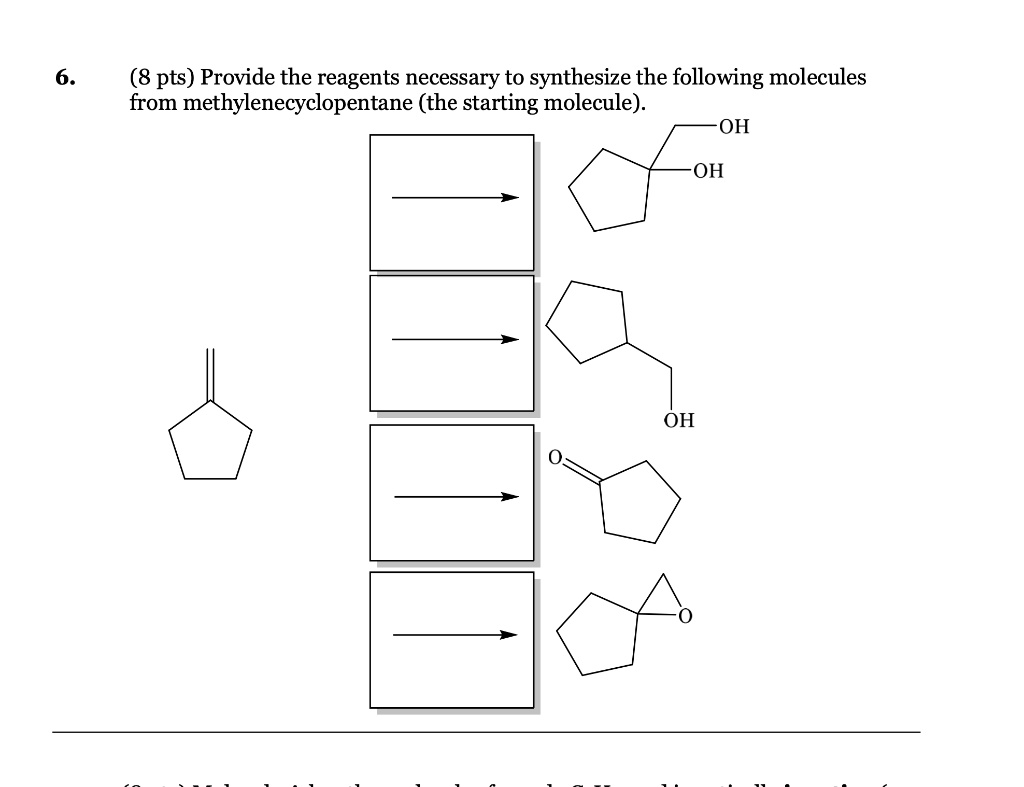
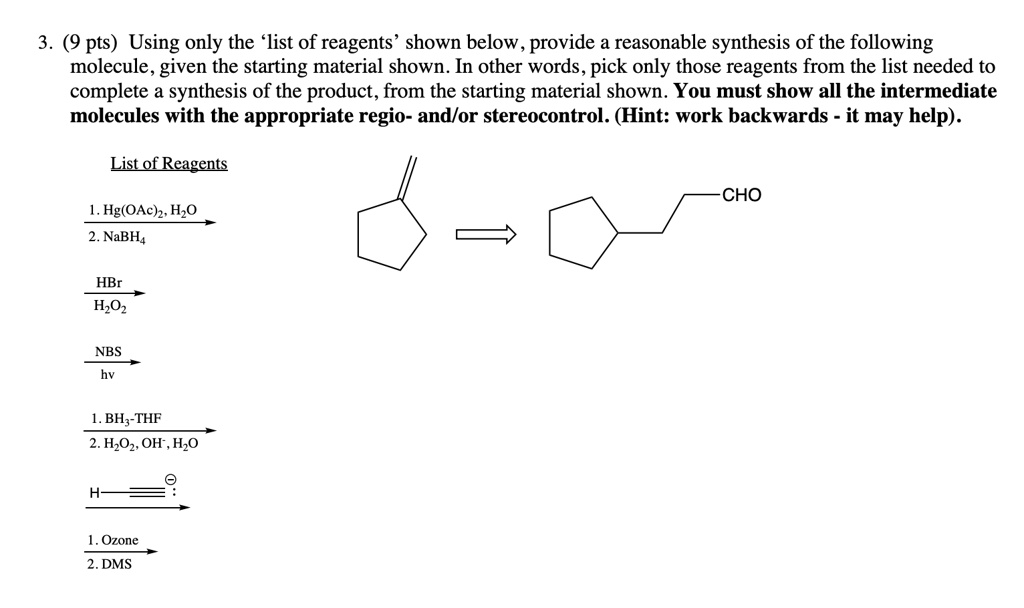
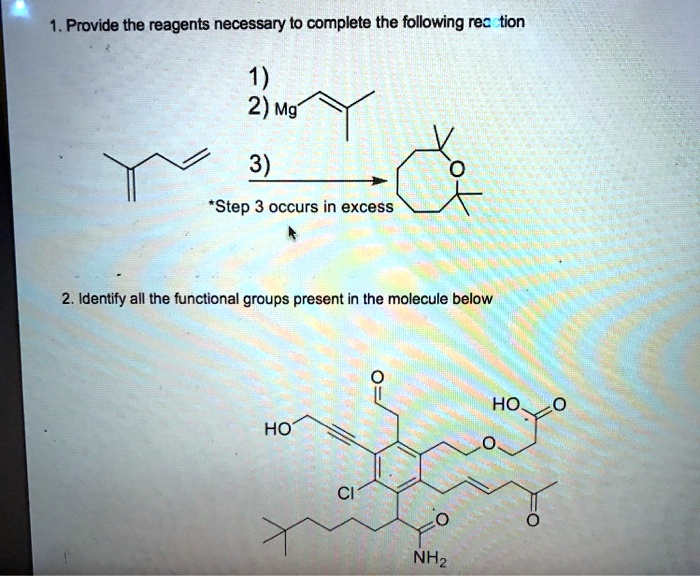
Provide The Reagents Necessary To Synthesize The Following Molecule.
Alright, buckle up, aspiring kitchen chemists and backyard alchemists! Today, we're diving headfirst into the thrilling, mind-bending, and dare I say, downright delicious world of making molecules. Think of it like baking, but instead of cookies, we're whipping up tiny, intricate structures that make up everything around us. It’s like having your own molecular LEGO set, but way cooler because these things do stuff!
We've got a special guest star today, a molecule so fascinating, so essential, it practically hums with its own awesomeness. It’s not going to cure all your ills or make you fly (yet!), but it’s a fundamental building block of life itself. And the best part? With the right ingredients, we can totally whip this bad boy up in our imaginary lab!
The Magic Ingredients List!
So, what do you need to conjure this molecular marvel into existence? Forget your dusty old textbooks filled with unpronounceable words. We’re talking about reagents that are, dare I say, almost… cute! These are the building blocks, the little helpers, the superheroes that will come together to form our grand creation.
First up, we need our foundational piece. Imagine it as the main stage for our molecular play. This essential character is none other than 1-bromo-3-methylbutane! Sounds fancy, right? But think of it as a four-carbon chain, like a tiny ladder, with a bromine atom hanging out at one end. Bromine is like the friendly waving hand, ready to connect with something new!
Now, our little ladder needs a dance partner. We can't just leave it hanging out all by itself. For this, we introduce magnesium! Now, you might think of magnesium as something you chew for a tummy ache, and you’d be partly right. But in the molecular world, this elemental powerhouse is ready to get its hands dirty, or rather, its electrons involved.
When our 1-bromo-3-methylbutane meets magnesium, something magical happens. It's like they're old friends meeting after a long time, and they decide to form a super-team. This super-team is known as a Grignard reagent. And this specific Grignard reagent is (3-methylbutyl)magnesium bromide. Ta-da! It’s like the molecule just got a glow-up and is ready for its close-up!
But our Grignard reagent, while impressive, is still a bit… reactive. It’s like a toddler after too much sugar – full of energy and ready to grab anything! So, to make it a little more stable and ready for the next step, we need something else. This next player is a classic: water! Yep, good old H₂O. It’s the ultimate mediator, the calming influence, the one who says, "Okay, let's all calm down and form something beautiful."
When our super-powered Grignard reagent meets water, it’s like a gentle handshake. The magnesium and bromine do their little tango, and then water steps in to help solidify the structure. This step is crucial, like letting your cake cool before frosting. It prevents things from getting messy and ensures our molecule is exactly how we want it.
The Grand Finale!
Now, we have our stabilized building block, ready for the final flourish. We need something to give our molecule its ultimate character, its signature scent, its reason for being! For that, we bring in a carbonyl compound. Think of carbonyl compounds as having a special double bond, a bit like a tiny, energized loop.
Our chosen carbonyl companion for today is acetone. You might know acetone as nail polish remover – a surprisingly powerful chemical that can dissolve some pretty stubborn stuff! But in our molecular kitchen, it’s a flavor enhancer, a structural contributor, the ingredient that adds that certain je ne sais quoi.
![Solved 13.[12 pts] Synthesize the following target molecules | Chegg.com](https://media.cheggcdn.com/media/f99/f99fc5c5-def0-4d91-9d4b-cb3bed9e78c2/image.png)
When our Grignard reagent, now nicely settled down, meets acetone, it’s a chemical explosion of happiness! The energized loop on acetone is just begging for our Grignard reagent to connect. It's like a perfect puzzle piece clicking into place. This is where the real creation happens!
The Grignard reagent happily latches onto the acetone, forming a beautiful, new connection. And just like before, we need our trusty friend, water, to finalize the process. A quick little splash of water, and our molecule is complete! It’s like adding the final sprinkle of glitter to a masterpiece.
And there you have it! With a dash of 1-bromo-3-methylbutane, a sprinkle of magnesium, a calming splash of water (twice, because sometimes things need a little extra love!), and a generous dollop of acetone, we’ve synthesized our magnificent molecule. It's a testament to the power of simple ingredients coming together to create something truly extraordinary. So next time you see something amazing, remember, it all starts with a few key players and a whole lot of chemical fun!
