Provide The Correct Systematic Name For The Compound Shown Here
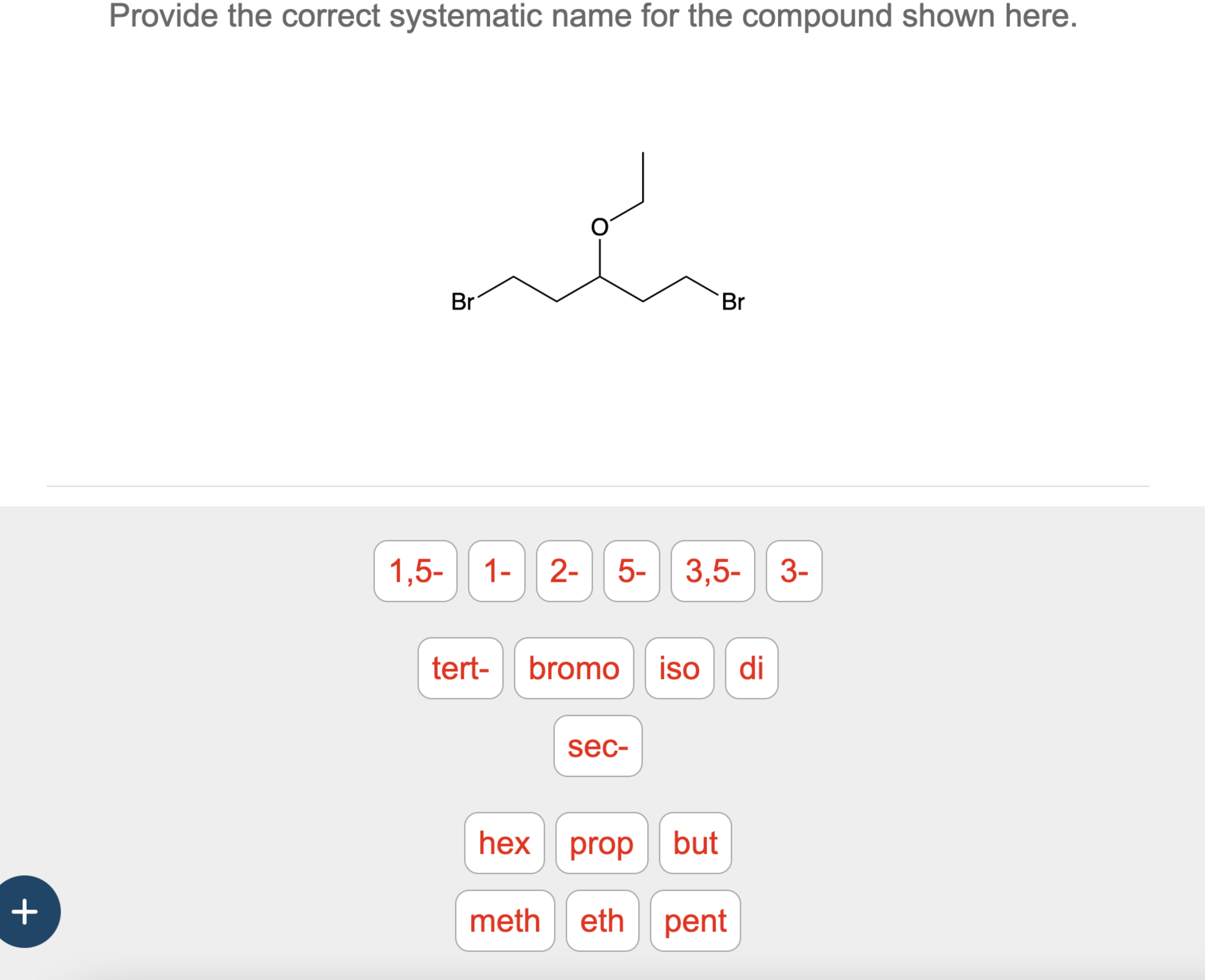
Hey there, coffee buddy! So, I was staring at this molecule the other day, you know, the kind that makes you squint a little. And I thought, "You know what? This deserves a proper introduction." We've all been there, right? Looking at something and thinking, "What IS that thing?" Well, that's what we're diving into today, just us, with our virtual mugs steaming. No stuffy lectures here, promise!
We're talking about giving this particular chemical compound its official name. The one they use in the textbooks, the one that’s supposed to tell you everything about it at a glance. It's like giving a nickname versus a full, proper title. You know, like calling your Uncle Bob "Uncle Bob" is fine, but his actual name, Bartholomew Reginald III, sounds a tad more… official, doesn't it? This is kinda like that, but with way more atoms and way fewer questionable fashion choices from the 70s.
So, what are we looking at here? Imagine a little skeleton, but made of atoms. And those atoms have little arms, called bonds, holding hands. Our job today is to become master name-givers, like chemical sheriffs in a wild, wild west of molecules. And the particular gem we've got our eye on today is… well, you’ll see it! It’s a bit of a… character.
The first step in this naming adventure is to find the longest carbon chain. Think of it as the main street of our molecular town. We gotta trace it, wiggle through all the twists and turns, and find the absolute longest path you can make just by hopping from one carbon atom to the next. It’s like a treasure hunt, but the treasure is a number, and the map is the molecule itself. Don't get distracted by the shiny side groups just yet; we're on a mission for the backbone!
Once you’ve found that longest chain – congratulations! You've got the parent name. It’s like the surname of our molecule. For example, if our longest chain has six carbons, then we’re talking about something that’s a derivative of hexane. Simple, right? Or if it's five, it's pentane. See? We're already halfway there. It’s like remembering your ABCs, but with a few more letters that have little hooks and loops.
Now, here’s where it gets a little more interesting. We need to look at what’s attached to that main chain. These are like the houses, shops, or maybe even that weird garden gnome collection on the side of the street. We call these substituents. They're the bits and bobs hanging off our main carbon highway. They can be all sorts of things – other carbon chains, halogens (like chlorine or bromine, the little troublemakers), or even oxygen atoms.
And each of these substituents gets its own little name, too. A one-carbon substituent? That's a methyl group. Two carbons? That's an ethyl group. Three carbons? You guessed it, propyl. It's like building blocks, and we're learning the names of each block. Easy peasy, lemon squeezy, right?
But wait, there's a twist! We can't just slap these names on anywhere. We need to tell everyone where they are. So, we have to number our main carbon chain. And here's the crucial part, the little rule that keeps everything from descending into chemical anarchy: we start numbering from the end that gives the substituents the lowest possible numbers. It’s like choosing the best starting point for a race, so everyone gets the fairest start. We want the little guys to have the smallest numbers, you know, to be closer to the beginning of the alphabet, so to speak.
So, let's say you have a methyl group on carbon number 2 when you count from one end, and carbon number 5 when you count from the other. Which end do we pick? The one that gives the methyl group the 2. Always go for the smaller number! It’s a fundamental principle of chemical politeness, really. We're being considerate of the substituents.
What if you have more than one of the same substituent? Like, two methyl groups chilling on the same chain? Well, we don't want to say "methyl methyl," that sounds a bit silly. Instead, we use prefixes! Di means two, tri means three, tetra means four. So, if we have two methyl groups, we'll say dimethyl. If there are three, it's trimethyl. You get the idea. It's like a chemical shorthand, saving us precious breath.
And of course, we need to specify their locations. So, if we have two methyl groups on carbons 2 and 3 of our main chain, we'd write 2,3-dimethyl. See? The numbers tell us exactly where they are, and the prefix tells us how many. It’s a beautifully organized system, really. Like a very precise filing cabinet for molecules.
What about different substituents on the same chain? Say, a methyl group and a chloro group? Do we just pick one to be more important? Nope! We put them in alphabetical order. So, a chloro group comes before a methyl group because 'c' comes before 'm' in the alphabet. Even if the chloro group is on carbon 5 and the methyl group is on carbon 2, it will be listed first in the name. It's a universal rule, folks. The alphabet reigns supreme!
So, if we had a methyl group on carbon 2 and a chloro group on carbon 4, the name would start with 4-chloro-2-methyl… (assuming that’s the correct numbering for the lowest numbers overall, of course!). It’s all about logic and order. No room for chaos in this molecular world!
Now, let’s zoom in on our specific molecule. Take a good look. See those carbons all linked up in a row? We've got to find that longest continuous chain of carbons. Don't be fooled by bends or twists! Follow it all the way. Can you see it? It's the backbone of our entire operation here. Give it a good count. How many carbons are there in that longest stretch? Pause for dramatic effect, imagine me holding up a chalkboard.
Once you’ve got the count, you know the parent name. If it’s six carbons, you're thinking hexane. If it's seven, heptane. You get the picture. This is the foundation of our molecule's identity.
Now, let's scan for those pesky substituents. What’s hanging off our main chain? Are there any little branches? Any atoms like chlorine or bromine acting as uninvited guests? What are they, and where are they located? Remember, we’re counting from the end that gives them the lowest numbers. This is the critical step, the one that separates the chemical amateurs from the seasoned pros!
So, for our compound, let's say we've identified the longest chain. Let's count those carbons carefully. And then, look at what’s attached. Aha! I see a little group there, a carbon with three hydrogens. That’s a methyl group. And where is it sitting on our main chain? Which carbon atom is it clinging to? Make a note of that number.
Now, are there any other substituents? Let's double-check. Sometimes they can hide in plain sight! If there are more, make sure you’ve got their positions noted down too. And if you have more than one of the same thing, don’t forget those prefixes: di, tri, tetra!
Let’s imagine, just for a second, that our longest chain has five carbons. So our parent name is pentane. And let’s say we found a methyl group hanging off the second carbon when we numbered from the correct end. What does that give us? It gives us 2-methylpentane!
But wait, what if there was another substituent? What if, in addition to that methyl group on carbon 2, there was also a bromine atom on carbon 3? Now, which one comes first alphabetically? Bromine, with its 'b', comes before methyl with its 'm'. So, we’d need to get the numbering right first, to ensure the lowest numbers overall. Let's assume the numbering is correct.
If the numbering gives us a methyl on carbon 2 and a bromo on carbon 3, and that's the lowest possible numbering scheme, then we'd put the bromo first alphabetically. So it would be 3-bromo-2-methylpentane.

It sounds like a mouthful, right? But that long, fancy name tells a chemist exactly what this molecule looks like, without even needing to see a picture. It’s a language all its own, and we’re learning to speak it, one syllable at a time!
So, for the compound you’re looking at, follow those steps. Find that longest carbon chain. Identify all the substituents. Note their positions, making sure you’ve numbered from the correct end to get the lowest numbers. And then, put it all together, alphabetically, with your prefixes if needed. Don't rush it! Take your time, have another sip of coffee. It's like putting together a puzzle, but the pieces are atoms, and the picture is a name!
And here it is, the grand reveal! For the compound shown, after carefully identifying the longest continuous carbon chain, which happens to be six carbons long, giving us the parent name hexane. Now, let’s look at the substituents. We have a methyl group hanging off the second carbon. And on the third carbon, we have… another methyl group! So, we have two methyl groups, which means we need our di- prefix.
We’ve got methyl groups on carbons 2 and 3. Since we’ve already confirmed this is the numbering that gives the lowest possible numbers, we can proceed. With two methyl groups, we combine that with the parent name. So, we have 2,3-dimethylhexane! Isn't that neat? It's specific, it's logical, and it tells you exactly what you're dealing with.
So there you have it, our molecule's official, systematic name. We've navigated the longest chain, counted our carbons, identified our little extras, and put them in their rightful places. It’s a little bit of detective work, a little bit of puzzle-solving, and a whole lot of chemistry fun. Who knew naming molecules could be this exciting? Now you can go forth and name with confidence! Just remember to keep those numbers low and the alphabet in order. Cheers!
