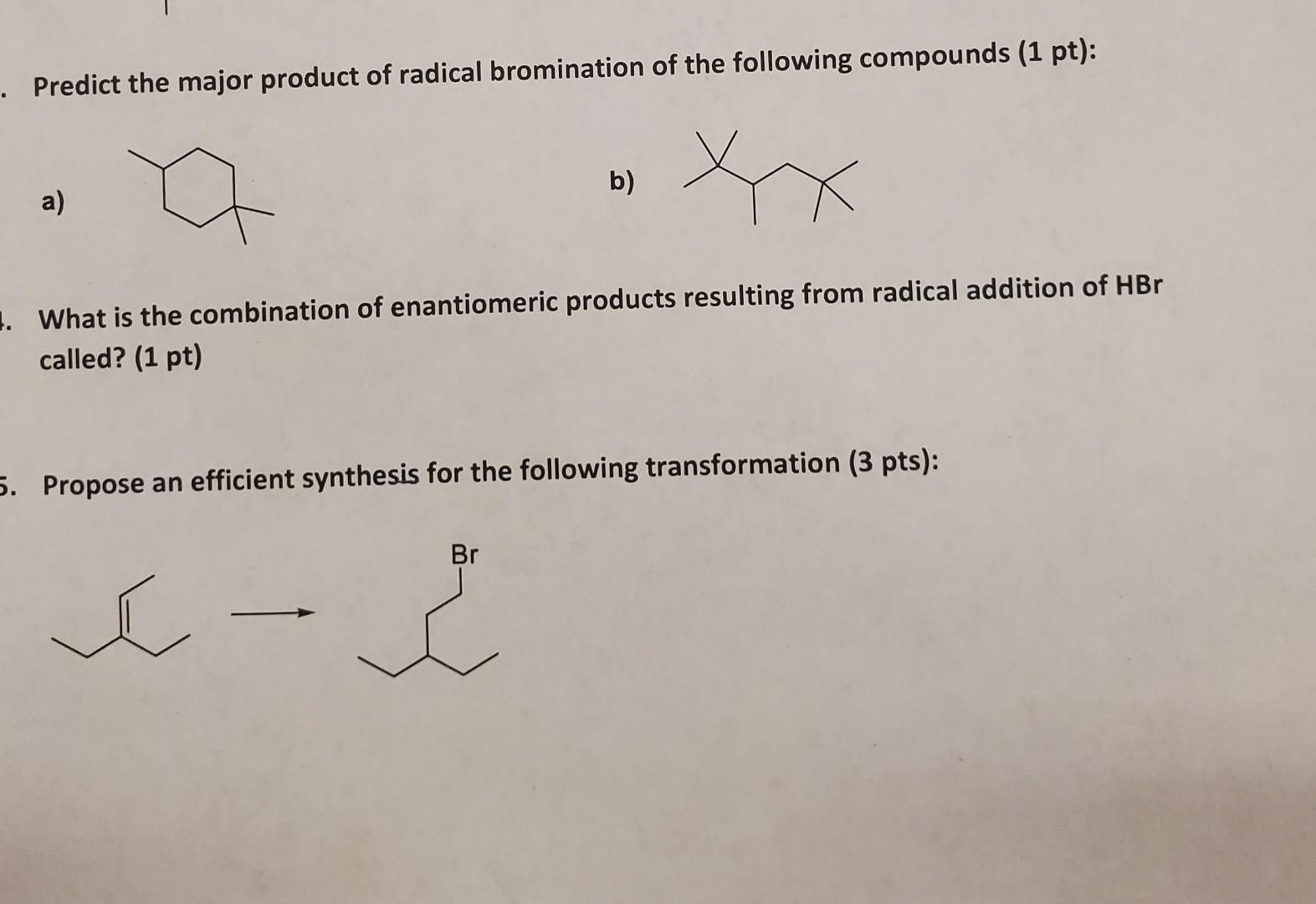
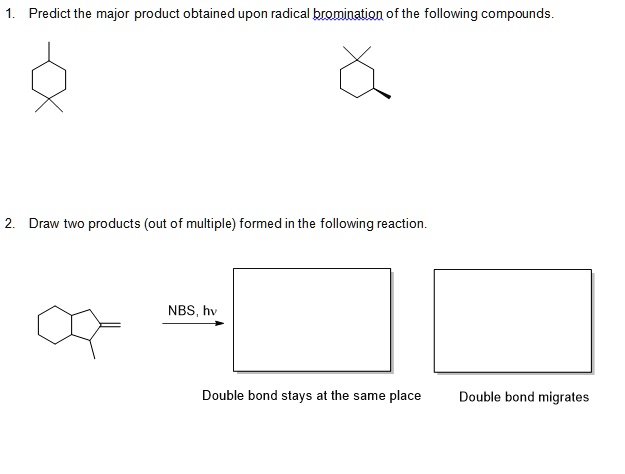
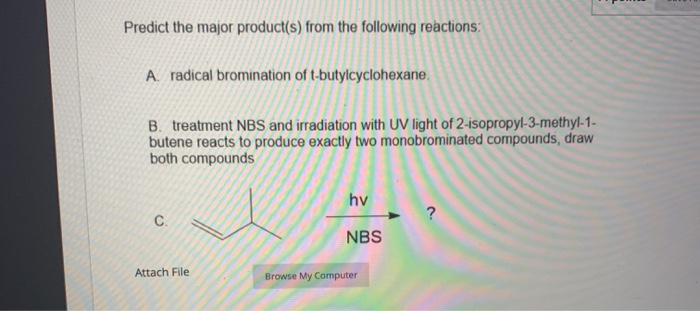
Predict The Major Product Of The Following Radical Bromination.
Hey there, science enthusiasts and curious minds! Ever feel like the world of chemistry is a bit, well, mysterious? Like there are secret codes and formulas that only super-nerds can crack? Today, I'm here to bust that myth wide open and show you how understanding a seemingly complex concept like radical bromination can actually be… dare I say it… fun!
Seriously! Imagine you're at a party, and someone throws out a chemical reaction. Instead of zoning out, you lean in and say, "Oh, radical bromination? Easy peasy! Let me tell you about the major product!" How cool would that be? It's like having a secret superpower, a little bit of intellectual sparkle that makes everyday conversations (or at least, very niche ones!) a whole lot more interesting.
So, what's this "radical bromination" thing all about? Don't let the fancy name scare you. At its heart, it's a way of selectively adding a bromine atom (that's Br for those in the know!) to a molecule, usually an alkane. Think of it like giving a specific part of a molecule a little makeover, a shiny new bromine accessory.
Now, the big question, the one that makes even seasoned chemists hum with excitement, is: which part of the molecule gets the bromine? This is where predicting the major product comes in, and trust me, it's like solving a puzzle. A puzzle that has real-world applications, too! From making medicines to creating new materials, understanding these chemical transformations is key.
The Nitty-Gritty (But Still Fun!)
Let's get down to brass tacks. Radical bromination typically happens in a few steps. First, you need to kick things off with something called an initiator, often light or heat. This breaks apart a bromine molecule (Br₂) into two super-reactive little guys called bromine radicals. These radicals are like tiny, energetic toddlers who just want to grab onto something!
Next, a bromine radical spots our unsuspecting alkane. This is where things get exciting! The radical wants to steal a hydrogen atom from the alkane. But not just any hydrogen atom. Oh no, the molecule has a bit of a preference!
This preference is all about something called stability. Imagine our bromine radical is looking for a place to land. It's going to choose the spot where it can feel the most comfortable, the most stable. And in the case of alkanes, the most stable places to form a new radical (which is what happens when the hydrogen is snatched) are at the tertiary carbons.
Tertiary, Secondary, Primary – What's the Diff?
Let's break down these terms, because they're super important for our prediction game. A primary carbon is attached to only one other carbon. Think of it as being on the outskirts of the molecular party. A secondary carbon is attached to two other carbons – a bit more in the thick of it. And a tertiary carbon? That's attached to three other carbons. It’s practically the life of the molecular party, the center of attention!
So, why do radicals prefer tertiary positions? It all comes down to something called hyperconjugation. Fancy word, I know! But think of it like this: the more carbon neighbours a radical has, the more they can "share" the unpaired electron. This sharing spreads out the electron's energy, making the radical more stable and therefore, more likely to form there. It's like having a bigger, more supportive group of friends when you're feeling a bit wobbly.

So, when our bromine radical goes on its hydrogen-snatching spree, it’s going to preferentially steal a hydrogen from a tertiary carbon if one is available. This forms a tertiary radical, which is the most stable. Then, a Br₂ molecule comes along and, poof, attaches a bromine atom to that spot, giving us our major product!
What if there are no tertiary carbons? Well, the radical will go for the next best thing: a secondary position. And if only primary positions are available? You guessed it, it'll grab a hydrogen from there, but this will be the minor product. It’s all about that stability hierarchy!
Let's Play a Little Game!
Let's say we have a molecule like 2-methylbutane. It looks a bit like this: CH₃-CH(CH₃)-CH₂-CH₃.
Now, let's identify our carbons. We have a primary carbon at one end (CH₃), a tertiary carbon where the methyl group is attached (the CH with the CH₃), a secondary carbon (CH₂), and another primary carbon at the other end (CH₃).

Where do you think the bromine is most likely to go? Drumroll please…
You got it! It’s going to attack that tertiary carbon. Why? Because it's the most stable spot to form a radical. So, the major product will be 2-bromo-2-methylbutane.
Pretty neat, right? You can look at a molecule, identify the different types of carbons, and predict where the action is going to happen. It's like having a crystal ball for chemistry!
Why This Matters (Beyond Party Tricks!)
This understanding of selectivity is huge in organic chemistry. It allows chemists to design reactions that produce the specific molecules they want, with minimal waste. Think about creating life-saving drugs – you don't want to accidentally make the "wrong" isomer, do you? This predictability is what makes chemistry a powerful tool for innovation.
Plus, it’s just plain satisfying to understand how things work on a molecular level. It’s like finally figuring out how a magic trick is done – the wonder might fade a little, but the appreciation for the cleverness grows exponentially!
So, the next time you encounter a radical bromination problem, don't groan. Smile! Because you now have the keys to unlock the mystery. You know to look for the tertiary carbons, then the secondary, and you can confidently predict the major product. You’re not just memorizing facts; you’re developing a chemical intuition.
And that, my friends, is where the real fun begins. This is just the tip of the iceberg, the first delicious bite of a much larger, incredibly fascinating feast. So, keep exploring, keep asking questions, and embrace the delightful complexities of the molecular world. You might just surprise yourself with how much you can discover and how much joy you can find in the process!
