Periodic Table Of Elements Brainpop Quiz Answers
Hey there, fellow knowledge-seekers! So, you've been diving into the wild and wonderful world of the Periodic Table of Elements, huh? Awesome! It's like a giant, organized cheat sheet for… well, everything, really. And if you've recently tackled that BrainPOP quiz – and let's be honest, who hasn't had a BrainPOP moment at some point? – you might be wondering about those answers. You know, the ones that made you scratch your head a little, or maybe do a little victory dance when you got them right. Don't worry, I've been there! Let's spill the tea on those BrainPOP Periodic Table quiz answers, shall we?
First off, how cool is the Periodic Table, anyway? It's like chemistry's ultimate lineup. All the elements, neatly arranged, telling us their secrets. It's not just a bunch of letters and numbers, people! It's a story. A story of building blocks. Think of it as the ultimate elemental family tree. And BrainPOP, bless their little animated hearts, tries to make it digestible. But sometimes, even the most enthusiastic explanation can leave you with a question or two, right? Especially when the quiz hits. Ugh, the quiz. The final boss of any BrainPOP adventure.
So, let’s break it down. What kind of questions are usually lurking in that quiz? They’re often testing your understanding of the basics. Like, what's an element? Is it just something you find in a science textbook, or is it the literal stuff that makes up… well, everything? That’s the kind of stuff they get you with. It’s the fundamental definition, the bedrock. You know, like how water is H2O? That’s two elements, hydrogen and oxygen, working together. Mind-blowing, right?
And then there’s the whole arrangement thing. Rows and columns. They’re not just pretty patterns, oh no. They tell you stuff! That’s where the terms periods and groups come in. Periods are the rows, and they tell you how many electron shells an atom has. Think of it like the atom's layers of clothing. The more periods you go down, the more layers. Simple enough, if you think about it like that. Maybe not that simple, but you get the idea. It’s a system, and systems are meant to be understood, not feared!
The groups, though! These are the columns, and they are so important. Elements in the same group? They’re like cousins, chemically speaking. They have similar properties. This is where the real magic of the Periodic Table happens. You see a new element in a group you already understand? Chances are, it'll behave in a similar way. It's like knowing that if you meet one grumpy cat, the next one in the same breed might also have a bit of an attitude. Same family, same vibe. It’s a scientific detective’s dream!

Common quiz questions will definitely revolve around identifying elements. You might see a symbol, like "Fe," and have to know it’s iron. Or you might see the name "Gold" and have to recall its symbol, "Au." Why "Au" for gold? Because of its Latin name, aurum. See? History and chemistry, besties again. It’s like a secret code you’re deciphering. And once you crack it, you feel like a super-spy. A super-spy of the atomic world.
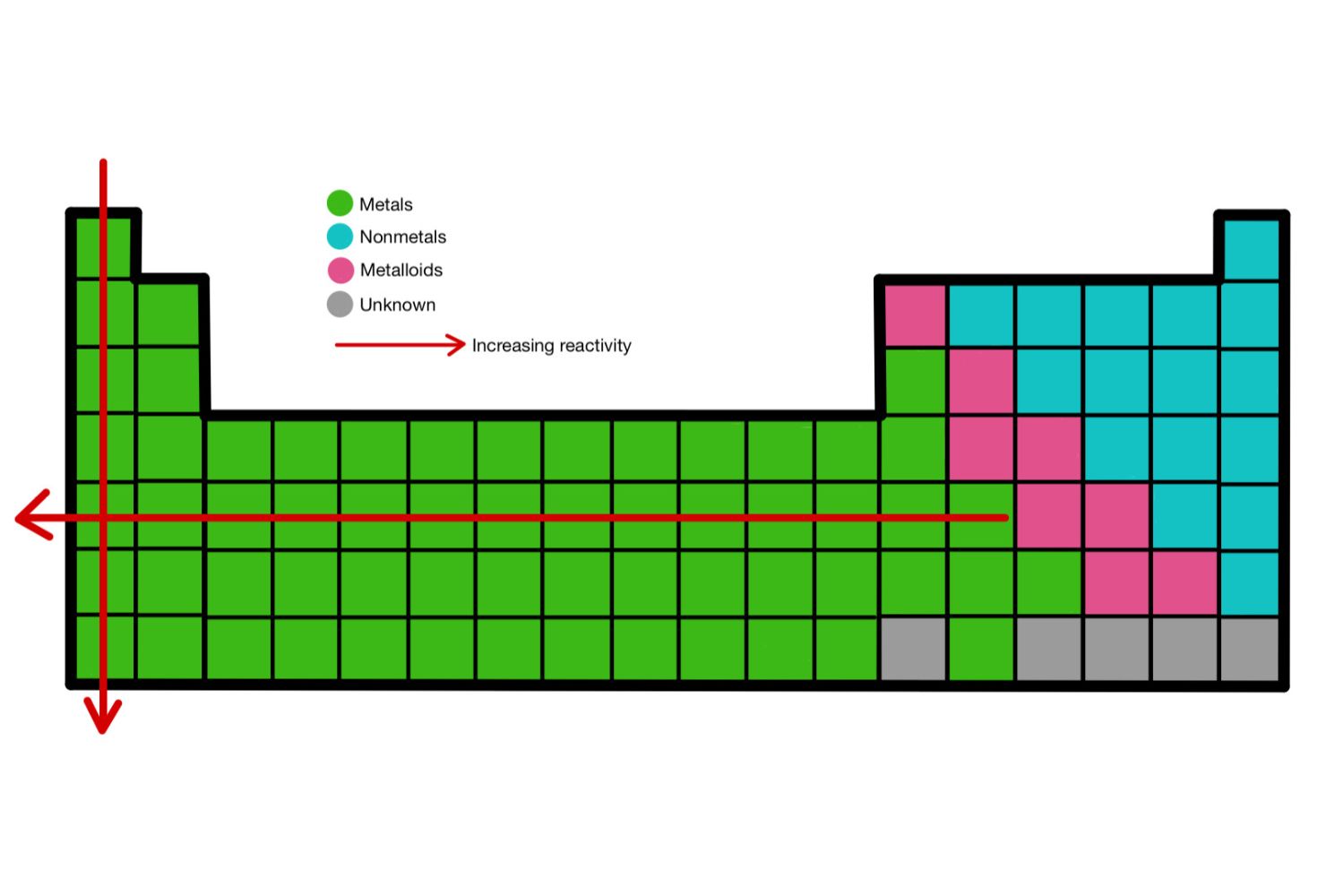
Then there are the categories of elements. This is where things get really interesting. You've got your metals, your nonmetals, and your metalloids. Metals are usually shiny, good conductors of heat and electricity, and they like to lose electrons. Think of your everyday stuff: spoons, wires, car parts. All metal. They’re the workhorses of the element world. And they make up the vast majority of the table, by the way. Surprise!
Nonmetals are, well, the opposite. They’re often dull, poor conductors, and they can gain or share electrons. Oxygen, carbon, nitrogen – these are all nonmetals. They’re crucial for life, though, so don't underestimate them! They might be the quiet ones, but they’re absolutely essential. It’s like the supporting cast in a movie. You need them for the whole story to work. And some of them are gases at room temperature! Can you imagine? Breathing in pure… something. Wild.

And the metalloids? They're the rebels. They're the fence-sitters. They have properties of both metals and nonmetals. Silicon, for example, is a metalloid and it's what makes your computer chips work. How cool is that? A bit of this, a bit of that, and suddenly you have the technology to watch more BrainPOP videos! It's a scientific balancing act. They bridge the gap, you know? Like a diplomat, but for atoms. Fascinating stuff.
Atomic number and atomic mass are also big players in the quiz game. The atomic number, which is just the number of protons in an atom’s nucleus, is like the element’s ID number. It’s unique to each element. No two elements have the same atomic number. It’s their definitive characteristic. And atomic mass? That’s roughly the number of protons plus neutrons. It gives you a sense of how heavy an atom is. Some of those heavier elements? They’re practically tiny, invisible bricks!
Don't forget the noble gases! These guys are the chill ones of the Periodic Table. They're in Group 18, and they're super stable. They don't really like to react with other elements. They're perfectly happy being alone. Think of them as the introverts of the atomic party. They've got their own vibe and they're not looking to mingle. Helium, neon, argon – they’re the cool, aloof characters. And they make balloons float! How’s that for practical?
Then you have the alkali metals (Group 1) and the alkaline earth metals (Group 2). These guys are way more reactive than the noble gases. Alkali metals, in particular, are very eager to give away an electron. So eager, in fact, that they can react explosively with water! Yikes! So, when you see questions about reactivity, remember these groups. They’re the high-energy, slightly dramatic members of the elemental family. Always the life of the party, or the cause of it.
Halogens (Group 17) are another interesting bunch. They’re one electron short of a full outer shell, so they’re super keen to grab an electron from someone else. They're the opposite of the alkali metals in a way, but just as reactive. Fluorine, chlorine, bromine – these are halogens. Chlorine, for example, is what’s in your swimming pool (thankfully, in a less reactive form). They're the element collectors, always looking for that perfect electron.
Sometimes the quiz might throw in questions about the discovery of certain elements. Who found what, and when? It’s like a history lesson woven into the science. It’s a reminder that these elements weren't always neatly laid out in a table. People worked hard, experimented, and discovered them over centuries. Imagine the excitement of finding a brand new element! Like discovering a hidden treasure, but it's the stuff that makes up everything.
And what about those isotopes? You might have seen that word pop up. Isotopes of an element have the same number of protons but a different number of neutrons. This means they have the same atomic number but different atomic masses. Carbon-12 and Carbon-14, for example. Both are carbon, but one has more neutrons. It’s like having identical twins who weigh slightly differently. It adds another layer of complexity, but it’s how things work in the real world.
So, when you're reviewing your BrainPOP Periodic Table quiz answers, don't just look at whether you got it right or wrong. Think about why. Did you mix up periods and groups? Did you forget the properties of alkali metals? Or maybe you just blanked on the symbol for potassium (which is "K" for the Latin kalium, by the way. Another Latin curveball!). Understanding the reasoning behind the answers is what really solidifies your knowledge. It's not just about memorizing; it's about understanding the underlying principles.
The Periodic Table is an ongoing story. New elements are still being discovered, and scientists are constantly learning more about the ones we already know. It’s a dynamic, evolving thing. So, don't feel discouraged if you didn't ace that quiz on the first try. It’s a huge topic! The goal is to keep learning, keep exploring, and keep asking questions. And hey, if you're ever in doubt, just remember that BrainPOP video. It's a great starting point, and those answers are out there, just waiting for you to connect the dots. Now go forth and conquer that elemental knowledge! You’ve got this!
