Oil In Water And Water In Oil: Complete Guide & Key Details

Ever looked at a salad dressing and wondered why the oil and vinegar didn't just do their own thing? Or maybe you've seen creamy lotions and thought, "How does that even work?!" It's all about a little bit of magic, or rather, science, that makes these everyday wonders happen. We're talking about oil and water, two things that really don't like to be buddies, deciding to hold hands and create something totally new.
Think of oil and water like two toddlers at a party. They're both having fun, but they definitely don't want to share toys or stand too close. Oil molecules are like shy, cliquey kids who prefer to stick with their own kind. Water molecules are a bit more outgoing, but even they find oil a bit… oily.
The Unlikely Duo: Oil and Water
So, why the big fuss? It all comes down to something called polarity. Water molecules are like tiny little magnets, with a positive end and a negative end. This makes them really good at attracting and dissolving other polar things, like salt or sugar.
Oil molecules, on the other hand, are like those super chill, laid-back friends who just want to float around and mind their own business. They're not polarized, meaning they don't have those positive and negative ends. Because of this, they can't really connect with water molecules. It's like trying to make a handshake work when one person has no hands.
When you try to mix oil and water, you'll see them stubbornly separate. It's like they're saying, "Nope, not today!" The oil will usually float to the top because it's less dense, creating those pretty, if somewhat annoying, layers. This is the natural state of things, the universe's way of keeping its oil and water in their respective corners.
When Oil Meets Water: Enter the Emulsifier!
But what if we want them to get along? What if we want that smooth, creamy texture of mayonnaise or the delightful blend of flavors in a vinaigrette? This is where our unsung heroes come in: emulsifiers.
An emulsifier is like a friendly matchmaker or a super-cool party planner. It's a special molecule that has two sides, and each side likes one of the ingredients. One side of the emulsifier loves oil, and the other side loves water. It's like a molecule with one hand reaching out to oil and the other to water.

When you add an emulsifier to oil and water and give it a good shake or stir (think of whisking vigorously!), the emulsifier molecules get busy. They surround the tiny droplets of oil, with their oil-loving side sticking to the oil, and their water-loving side pointing outwards towards the water. This creates a protective barrier around each oil droplet.
It's like the emulsifier is building tiny little force fields around the oil particles, preventing them from clumping back together and forming a solid layer. The water molecules can then happily coexist with these protected oil droplets. This is how we get emulsions.
Imagine a crowd of people (water) at a concert, and a bunch of people in special rainbow shirts (emulsifiers) come in. These rainbow-shirt people then grab the hands of a group of people wearing sunglasses (oil), forming little circles. The sunglasses people can't get too close to the concert-goers anymore, but everyone can still be in the same space.
The Two Main Types: Oil-in-Water and Water-in-Oil
Now, depending on how many of our ingredients we have and how the emulsifier is doing its job, we get two main types of emulsions. It’s like choosing between two different party themes.
Oil-in-Water (O/W) Emulsions: The "More Water" Party
In this type, there's a lot more water than oil. The tiny oil droplets are dispersed throughout the water. Think of it like a few shy oil molecules hiding in a big pool of water, all nicely tucked away by our emulsifier friends.

This is your classic salad dressing, like a light vinaigrette. It’s not super thick or creamy, and it often separates over time if left undisturbed. You might see the oil gathering at the top again, giving you a little nudge to give it a good shake.
Many lotions and shampoos are also oil-in-water emulsions. They feel light and refreshing on your skin, and they spread easily. It's the kind of product that makes you feel clean and moisturized without feeling greasy.
The magic of lecithin, found in egg yolks, is a fantastic example of a natural emulsifier that makes O/W emulsions. It’s why mayonnaise is so wonderfully smooth and creamy, with those little oil droplets holding hands with the water!
Water-in-Oil (W/O) Emulsions: The "More Oil" Party
In this case, there's more oil than water. The water droplets are dispersed within the continuous oil phase. It's like a bunch of water balloons floating in a big ocean of oil, all safely contained.
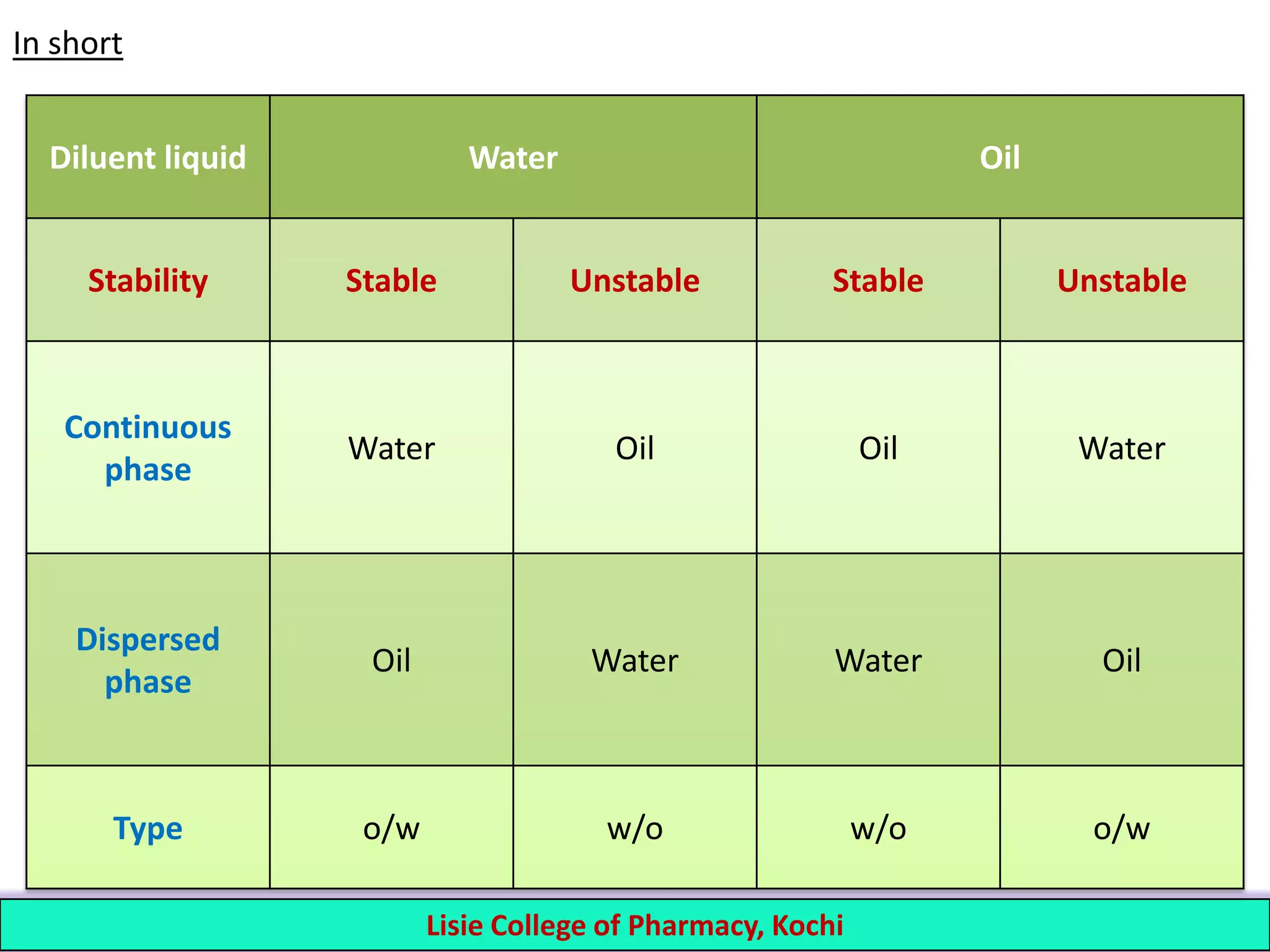
Butter and margarine are perfect examples of water-in-oil emulsions. They feel richer and more solid because the oil is the dominant ingredient. That delightful creaminess comes from those tiny water droplets being spread throughout the fatty goodness.
Some thicker creams and certain types of makeup are also W/O emulsions. They can feel a bit heavier on the skin, providing a more intense moisturizing effect. It’s like giving your skin a cozy blanket.
The key here is that the emulsifier helps to keep the water from merging and forming larger pockets. It's a delicate balance, but when it works, it's pure genius!
Everyday Wonders and Humorous Mishaps
These emulsions are all around us, making our lives tastier and more convenient. From the frosting on a cake to the sunscreen on your nose, you're interacting with emulsions every single day. It's a quiet revolution happening in your kitchen and your bathroom!

Sometimes, though, emulsions can be a bit temperamental. If you don't add enough emulsifier, or if you add too much of one ingredient, things can go wrong. That's when your beautiful salad dressing might separate completely, leaving you with oily goo on top.
Or perhaps your homemade mayonnaise decides to split, looking more like a curdled mess than a creamy delight. It can be a little disheartening, but think of it as a friendly reminder from science that even the best relationships need the right support. A little extra whisking or a pinch more emulsifier might just save the day!
The beauty of it all is that with a little understanding and the right ingredients, we can manipulate these seemingly stubborn substances. We can make oil and water, the ultimate opposites, work together in harmony. It's a testament to how clever nature (and sometimes, human ingenuity) can be.
So next time you're enjoying a creamy sauce or a perfectly blended smoothie, take a moment to appreciate the unsung heroes: the emulsifiers. They're the little molecules working overtime to bring oil and water together, creating the delightful textures and flavors we often take for granted. They are the true peacemakers of the culinary and cosmetic world!
