Net Ionic Equation For Sodium Phosphate And Barium Chloride
Get ready for a splash of chemistry fun! Today, we're diving headfirst into the electrifying world of net ionic equations, and our star performers are none other than sodium phosphate and barium chloride. Don't let those fancy names intimidate you; think of them as ingredients in a super cool, albeit invisible, kitchen experiment. We're going to whisk them together and see what magical (and messy!) reactions occur.
Imagine you're at a dazzling party, and you've got two very popular guests: Sodium Phosphate, a total crowd-pleaser, always happy to mingle and dissolve. And then there's Barium Chloride, another charmer, equally eager to join the fun and break apart into its individual, energetic ions. They're like the life of the party, ready to dance and mingle with anyone who comes their way.
When these two party animals meet in a solution – which, in chemistry terms, means they're dissolved in water, like a refreshing drink – something truly spectacular happens. They don't just politely shake hands; oh no! They go through a complete, dramatic, and utterly thrilling breakup! Sodium Phosphate splits into happy little bits called sodium ions and phosphate ions. And Barium Chloride? It does the same, breaking into eager barium ions and chloride ions. It's like a confetti cannon going off, but with charged particles!
Now, here's where the real excitement kicks in. These individual ions are on the hunt for new dance partners. The sodium ions and the chloride ions? They're perfectly happy to stay dissolved, like a couple who've already found their groove on the dance floor and are just chilling. They're spectators, enjoying the show but not actively participating in the big finale. They're like the background dancers, just vibing.
But the barium ions and the phosphate ions? They have a different destiny! They look at each other, and BAM! Instant attraction. They decide to ditch their old partners and form a brand-new, super-stable compound. This new compound is called barium phosphate. And this isn't just any old compound; it's so incredibly happy and stable that it decides it doesn't want to be dissolved in the water anymore. It's like they've found their soulmates and want to build a cozy little house together, away from the bustling party.
What does this mean for our experiment? It means this barium phosphate stuff starts to clump together. It becomes solid, like little microscopic boulders forming at the bottom of our beaker. It precipitates! Think of it as the most exclusive VIP section of the party, where only the happiest, most stable couples are allowed. The rest of the ions are still mingling, but these two have found their forever.
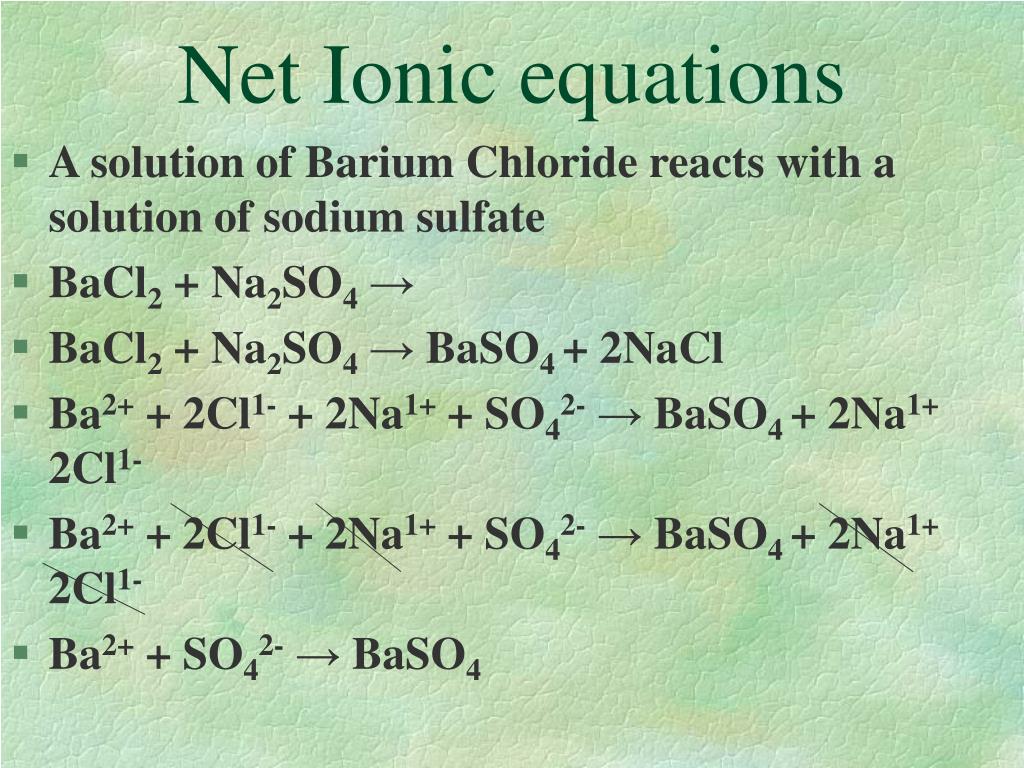
So, what's the big deal about the net ionic equation? It's like we're cutting through all the chatter and the background noise of the party. We're focusing only on the ions that actually do something, the ones that are involved in the real action – the forming of that solid, beautiful barium phosphate. It's the highlight reel, the epic showdown, the part of the story that truly matters!
We start with our initial ingredients, right? We've got our sodium ions (Na⁺) and phosphate ions (PO₄³⁻) chilling from the sodium phosphate. And from the barium chloride, we've got our barium ions (Ba²⁺) and chloride ions (Cl⁻). They're all floating around, minding their own business, ready for whatever happens next.

But then, the magic unfolds. The barium ions and phosphate ions decide to team up. They go from being separate, floating individuals to a solid, united front of barium phosphate (Ba₃(PO₄)₂). This is the grand finale! The spectators, our sodium ions and chloride ions, are still there, still dissolved, but they're not part of this solid formation. They're just like, "Oh, look at them go!"
The net ionic equation is our way of showing this amazing transformation in its purest form. It strips away the ions that just watch from the sidelines and highlights the star performers: the ones that actually form the solid precipitate. It's like zooming in on the most dramatic moment of a movie and ignoring all the filler scenes. We're left with the essence of the reaction, the core event that changed everything.
So, when you see the net ionic equation for sodium phosphate and barium chloride, you're witnessing the birth of barium phosphate. You're seeing how different parts of matter can come together, break apart, and form something entirely new and wonderfully solid. It's a testament to the incredible, dynamic nature of the world around us, happening right there in our beakers (or our imagination!). Pretty neat, huh? It's chemistry in action, and it's more fun than a barrel of monkeys... or a beaker full of precipitating barium phosphate!
