Net Ionic Equation For Lead Ii Nitrate And Potassium Iodide

Get ready for some chemistry magic, folks! Today, we're diving into a reaction that’s like a culinary explosion, but with way less mess and a lot more sparkle. Imagine you're a mad scientist in your kitchen, whipping up something truly… elemental. We're talking about mixing two seemingly innocent liquids and watching them do a spectacular disappearing act.
Our star players today are a pair of really fancy-sounding chemicals: Lead(II) Nitrate and Potassium Iodide. Now, don't let those big names scare you. Think of them as our celebrity chefs for this particular chemical cook-off. They’ve got their own unique personalities and when they meet, oh boy, do they make a show!
First up, we have Lead(II) Nitrate. Imagine it’s a sophisticated party guest, all dressed up in its sparkly, clear, aqueous attire. It’s dissolved in water, ready to mingle and be fabulous. It's like that friend who shows up to every party looking impossibly chic and bringing the best appetizers.
Then, sashaying in with equal flair, is Potassium Iodide. This one’s also ready to party, happily dissolved in its own watery entourage. It’s the vibrant, energetic friend who always brings the good music and infectious laughter. They’re both ready for a good time!
Now, here comes the moment of truth! We pour these two together, and what do you think happens? Do they just politely say hello and remain the same? Oh, heavens no! That would be dreadfully boring, wouldn't it? This is where the real excitement kicks in, and our chemical concoction decides to put on a show.
As soon as Lead(II) Nitrate and Potassium Iodide get acquainted in the same beaker, they start to get… a little too excited. They're like those two friends who haven't seen each other in ages and decide to spontaneously break out into an impromptu dance-off. It's a flurry of chemical activity, a whirlwind of atoms.
And then… BAM! Out of nowhere, something completely unexpected appears. It’s not a party favor, it’s not a new song, it’s a brand new substance! This new substance is so different, so distinct, that it can’t even be bothered to stay dissolved. It decides to become a solid, forming beautiful, bright yellow particles.

These tiny, vibrant yellow specks are called a precipitate. Think of it as the chemical equivalent of a confetti cannon going off – but way cooler because it’s made of actual chemistry! It’s a visual declaration that something truly epic has just happened.
This particular precipitate is a celebrity in its own right: Lead(II) Iodide. It’s the showstopper, the main event that makes everyone gasp. It’s that perfect moment when the fireworks go off and light up the entire sky in a magnificent display of color.
But what about the other characters in our chemical drama? Well, they don't just vanish into thin air, that would be irresponsible! The Potassium ions and the Nitrate ions from our original ingredients are still there, floating around happily in the water. They decided this whole yellow precipitate thing wasn't really their scene, so they just chilled out.
They are the spectators at the dance-off, cheering from the sidelines. They’re the friends who chose to have a quiet chat rather than join the energetic party. They’re still part of the mix, but they’re not actively participating in the dramatic formation of the yellow stuff.
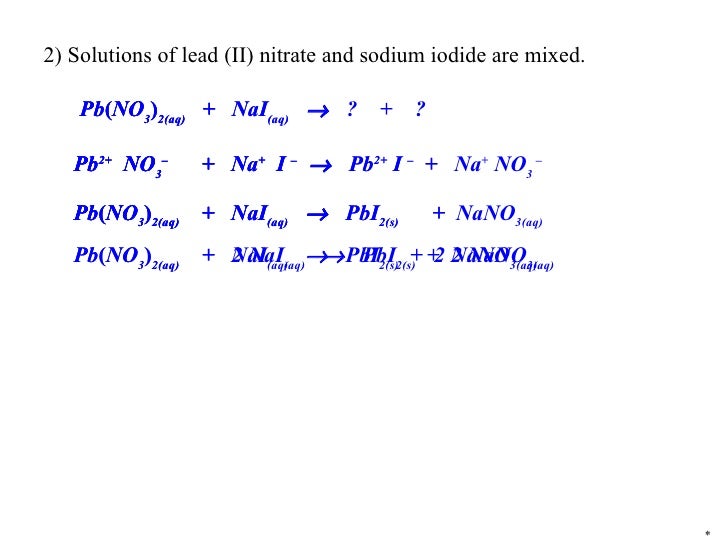
So, we started with two clear solutions, and ended up with a vibrant yellow solid suspended in a clear solution. It's a transformation worthy of a superhero origin story! This isn't just some abstract concept; it’s a tangible, visual representation of chemical reactions happening right before your eyes.
Now, in the grand theater of chemistry, we have different ways of describing these spectacular events. We have the full, detailed script, which we call the molecular equation. This shows absolutely everything that’s going on, every single atom and molecule involved, like a director’s cut with all the behind-the-scenes action.
But sometimes, we want to focus on the real stars of the show, the ones who are actually making the magic happen. We want to highlight the dramatic transformation, the appearance of that dazzling yellow Lead(II) Iodide. For this, we use something called the net ionic equation.
Think of the net ionic equation as the highlight reel. It cuts out all the characters who were just standing around, the ones who didn’t really change or form anything new. It focuses only on the participants who are actively involved in the formation of the new, solid substance – our amazing Lead(II) Iodide.
It’s like watching a movie trailer. You don’t see every single scene, but you get all the best action, the most exciting moments. The net ionic equation does the same for chemistry; it gives you the punchline, the grand reveal, the reason for all the fuss!

So, to sum up this delightful chemical adventure, we witnessed Lead(II) Nitrate and Potassium Iodide doing their thing. They mixed, they mingled, and then poof! A magnificent yellow precipitate of Lead(II) Iodide appeared. The Potassium and Nitrate ions decided to be the chill observers, not getting involved in the main event.
The net ionic equation is our way of saying, "Okay, these are the guys who really mattered in this particular chemical dance." It’s the simplified version that shows us the essential players and their transformative actions. It’s the essence of the reaction, distilled into its most potent form.
Isn't that just the coolest? We take some everyday liquids, add a dash of scientific curiosity, and voilà – a brilliant yellow solid appears! It’s a testament to the wonders of the universe, happening in a beaker, and we get to peek behind the curtain. So next time you see a clear liquid turn into something spectacularly solid and colorful, you'll know it's probably a chemical party you're witnessing!
The beauty of the net ionic equation is that it strips away the unnecessary chatter and gets straight to the heart of what's happening. It’s the ultimate shortcut to understanding the core chemical drama. It’s like knowing the secret handshake of the chemical world!
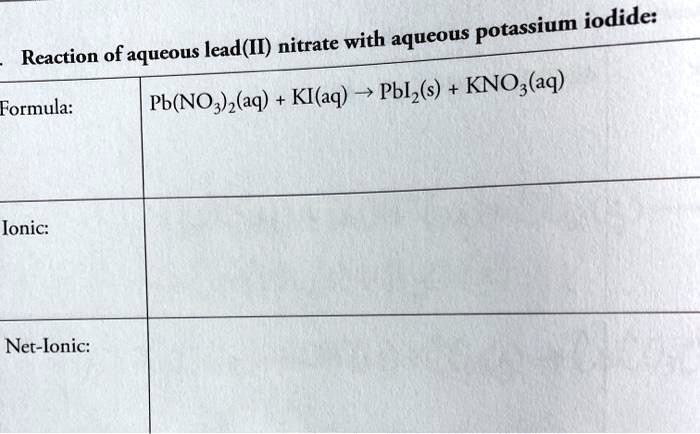
So, there you have it! A splash of Lead(II) Nitrate, a dash of Potassium Iodide, and a whole lot of dazzling Lead(II) Iodide precipitate. The net ionic equation simply highlights the main actors in this fascinating chemical play. Keep exploring, keep questioning, and keep enjoying the marvelous world of chemistry!
Remember, the net ionic equation is your VIP pass to understanding the most exciting parts of a chemical reaction. It’s where the real action happens, leaving all the spectators behind!
It's a reminder that even in the seemingly simple act of mixing liquids, there's a whole universe of atomic interactions and transformations waiting to be discovered. And that, my friends, is something to get seriously excited about!
The next time you're feeling a little low, just remember the dramatic flair of Lead(II) Iodide forming. It’s a tiny, yellow reminder that amazing things can happen when elements decide to get together. And who knows, maybe you'll be inspired to create your own chemical magic!
We’ve unveiled the secret behind that vibrant yellow hue, and the net ionic equation is our key to unlocking that understanding. It’s a simple yet powerful tool for appreciating the fundamental processes that shape our world. So go forth and be chemically curious!
