Monosaccharides Are Characterized By All Except Which Of The Following
So, I was recently rummaging through my grandma's old recipe box – you know, the one with the faded floral print and that faint scent of vanilla extract? I stumbled upon this incredibly intricate cake recipe from the 1950s. It called for all sorts of fancy ingredients, some of which I had to Google, obviously. But what really caught my eye was this tiny, almost illegible note scribbled in the margin next to the list of sugars: "Remember, it's the simple ones that make all the difference." At the time, I just nodded sagely, pretending I understood the profound baking wisdom. Turns out, Grandma wasn't just talking about cake; she was practically dropping hints about
Funny how life works, right? You think you're just leafing through old recipes, and suddenly you're on a deep dive into the fundamental building blocks of sweet stuff. And honestly, who doesn't love a bit of sweet stuff? Whether it’s the sugar in your morning coffee, the fruit in your smoothie, or, yes, that ridiculously decadent cake Grandma was so keen on, we're constantly interacting with these tiny carbohydrate powerhouses. But what exactly makes them tick? What defines these fundamental sugar units?
This is where we get to the juicy bit. We're talking about
Now, this is where the real detective work begins. We're going to play a little game of "Which One Doesn't Belong?" You know, like those worksheets you used to get in elementary school, but with a whole lot more chemistry involved. We're going to look at the characteristics that define these amazing little molecules, and then identify the one thing that doesn't fit the bill for a monosaccharide. It's all about understanding what makes them, well, them.
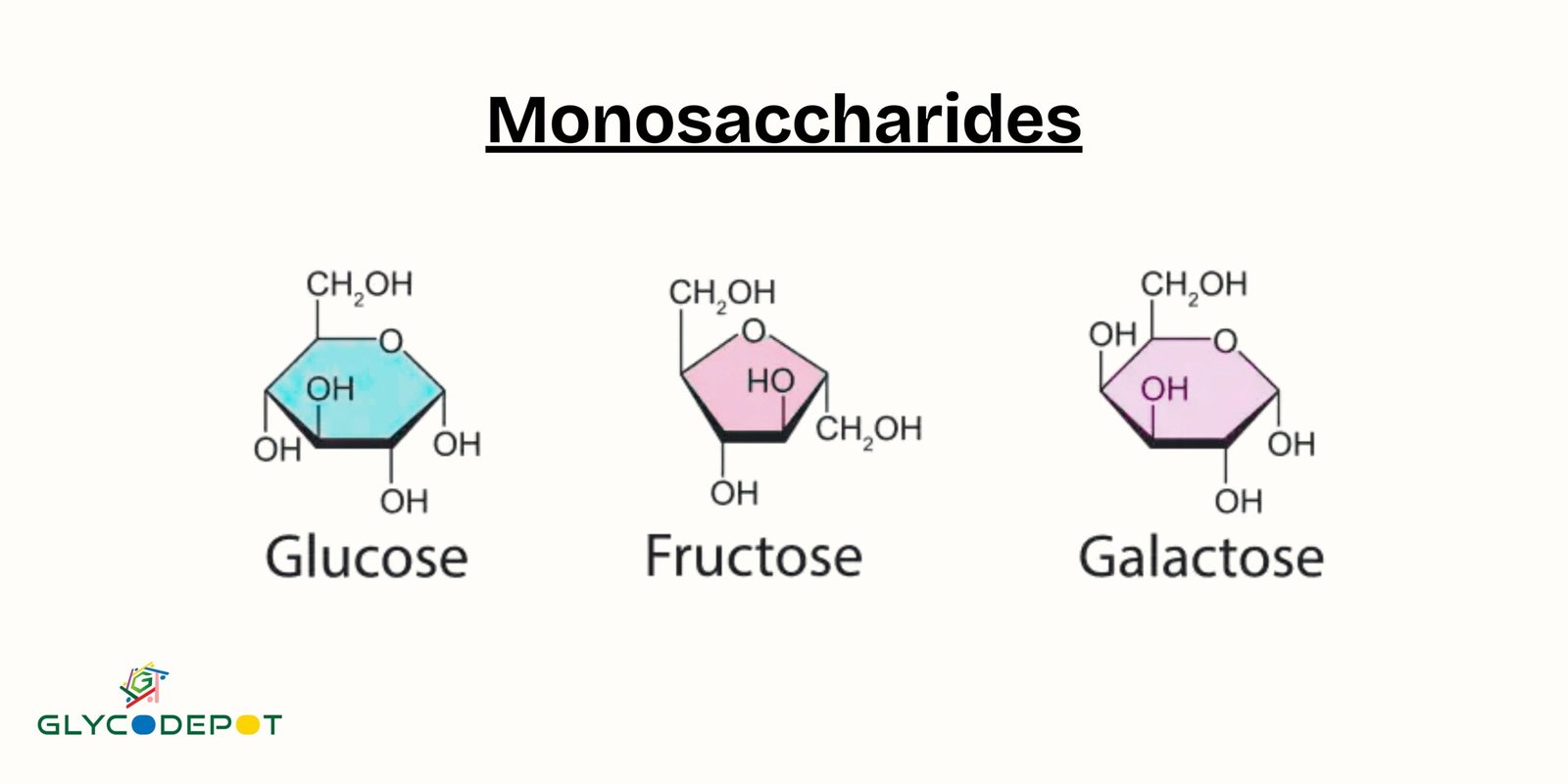
The Hallmark Traits of a Monosaccharide
So, what are the defining features, the undeniable qualities that scream "I'm a monosaccharide!"? Let's break them down:
1. They are the Simplest Sugars
This is pretty much their raison d'être. As we touched on, they are the foundational sugars. They are not formed by the glycosidic linkage of smaller sugar units. If you’re talking about a carbohydrate, and it can be broken down into smaller carbohydrate units through hydrolysis, then congratulations, it's not a monosaccharide. It's something more complex, like a disaccharide (two monosaccharides joined) or a polysaccharide (many monosaccharides linked together).
Think of it this way: glucose is a monosaccharide. Sucrose (table sugar) is a disaccharide because it's made of glucose and fructose. Starch? That’s a polysaccharide, a massive chain of glucose units. So, the "simple" aspect is paramount. They are the ABCs of the carbohydrate alphabet. You can't have words without letters, and you can't have complex sugars without these basic units.
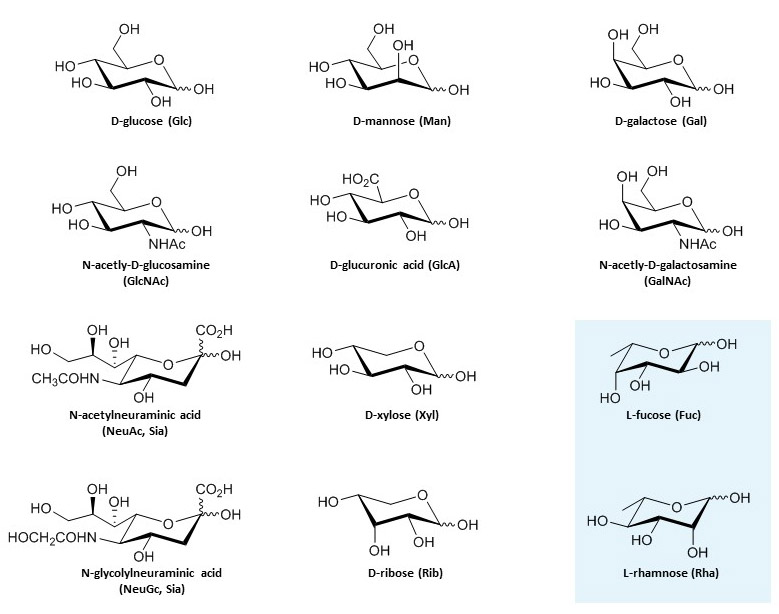
2. They Possess a Carbonyl Group
Every monosaccharide has a carbonyl group (
This distinction is actually quite important in carbohydrate chemistry. Monosaccharides with an aldehyde group are called
3. They Have Multiple Hydroxyl Groups
Along with that all-important carbonyl group, monosaccharides also sport multiple
These hydroxyl groups are super important for their solubility in water (which is why many simple sugars dissolve easily in your tea or coffee – your grandma was probably thinking about this too!). They also participate in hydrogen bonding, which is a pretty fundamental force in chemistry. So, the more -OH groups, the more opportunities for these interactions. It's like having more arms to hug other molecules!
4. They Have a Specific Number of Carbon Atoms
Monosaccharides are classified by the number of carbon atoms they contain. The smallest monosaccharides have three carbon atoms, and these are called
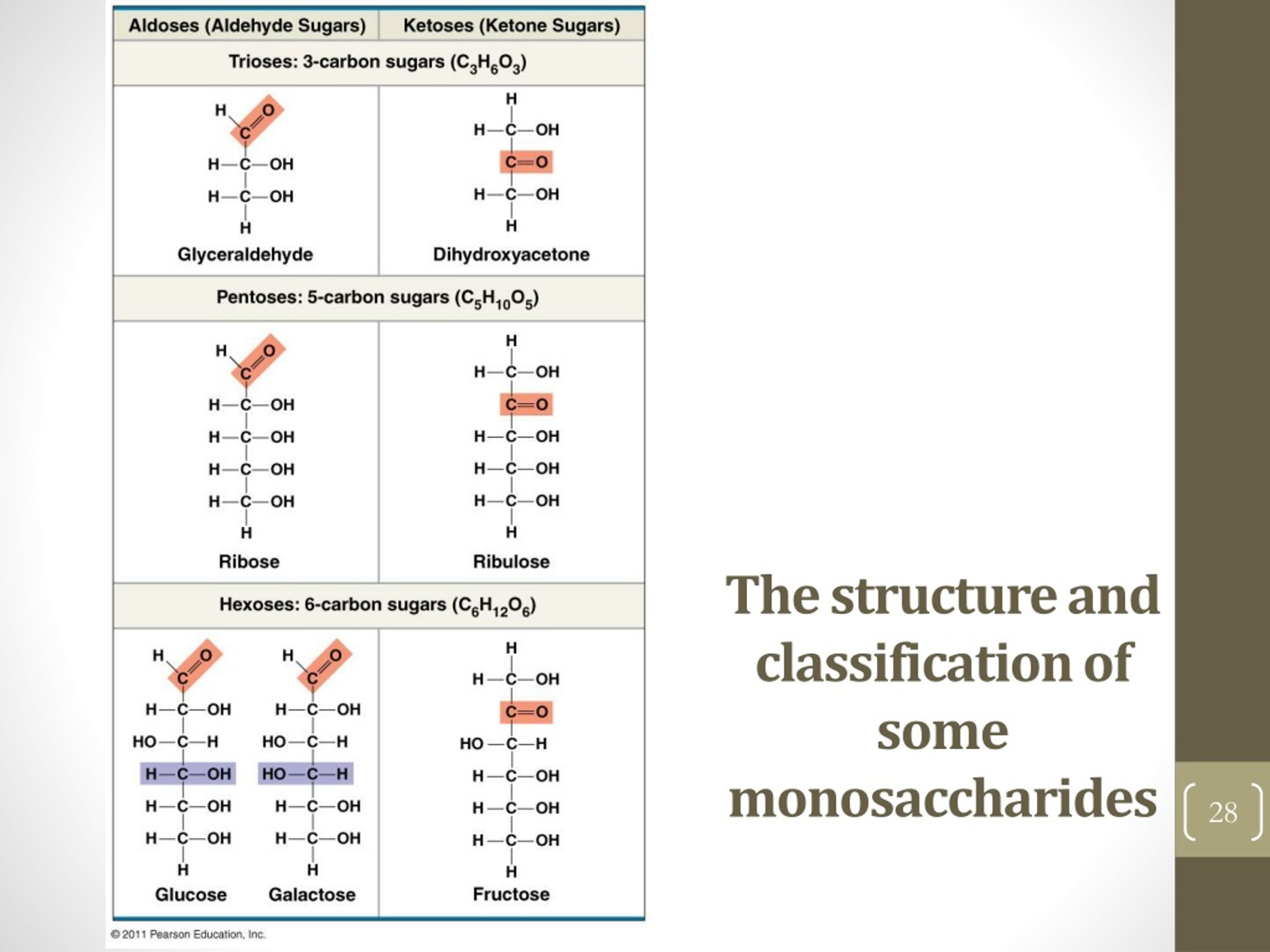
So, while they can have varying lengths of carbon chains, there's a clear structure based on this count. It's not like they can have an infinite number of carbons; there are practical limits dictated by their stability and how they can fold and interact. It’s like building with LEGOs – you can make a small car or a big truck, but you’re still using the same basic bricks.
Now, Let's Play "Find the Odd One Out"!
We’ve laid out the key characteristics. Now, let's think about what could be a curveball. What’s something that sounds like it might fit, but actually disqualifies a molecule from being a monosaccharide? Let’s ponder some possibilities. Remember, we’re looking for the characteristic that isn't true for all monosaccharides.
Could it be their solubility in water? We mentioned the hydroxyl groups contribute to this, and generally, monosaccharides are quite water-soluble. So, that’s probably a characteristic, not an exception. What about their sweetness? While most are sweet, not all are intensely so, and taste isn't exactly a scientific defining feature. Plus, some non-sugars can be sweet.
What about their ability to form rings? Ah, this is an interesting one. In solution, monosaccharides with five or more carbons tend to cyclize, meaning they fold up and form ring structures. Glucose, for instance, exists predominantly as a cyclic molecule in aqueous solution. So, while they can exist in an open-chain form, their tendency to cyclize is a very common and important behavior. This isn't an exception to being a monosaccharide, but rather a characteristic behavior they exhibit.
Let's think more fundamentally. We've talked about the carbonyl group, the hydroxyl groups, the carbon backbone. What if we consider their ability to be reduced or oxidized? Monosaccharides are reducing sugars (meaning they can reduce other compounds, often due to the aldehyde group). This is a pretty key chemical property. So, that’s likely a characteristic, not an exception.

This is where we need to be really precise. We're looking for something that, if present, would mean it's not a monosaccharide. Or, conversely, something that's absent in all monosaccharides.
Let's consider the options that often come up in these kinds of questions:
- Possessing a carbonyl group. (Essential!)
- Having multiple hydroxyl groups. (Essential!)
- Being a simple, indivisible sugar unit. (Essential!)
- Containing nitrogen atoms. (Hmmmm...)
- Having a carbon backbone with a specific number of carbons. (Essential!)
Now, let’s zoom in on that nitrogen atom idea. Think about the general formula for carbohydrates:
So, if a molecule contains nitrogen atoms as part of its core structure, it's very likely not a pure monosaccharide. It might be an amino sugar, which is a modified monosaccharide, or something entirely different. But for something to be classified as a monosaccharide, it needs to stick to that carbon-hydrogen-oxygen makeup, with the defining carbonyl and hydroxyl groups.
The Decisive Factor: The Absence of Nitrogen
Therefore, when you're asked what characterizes monosaccharides, and then presented with options, the one that will not be a characteristic is the presence of nitrogen. Monosaccharides are defined by their simple structure, their carbonyl group (aldehyde or ketone), their multiple hydroxyl groups, and their specific carbon chain length. They are, in essence, pure carbohydrates. The inclusion of other elements like nitrogen fundamentally changes their classification.

It’s like if Grandma’s recipe said, "Add one cup of flour, two eggs, a pinch of salt, and… a small, perfectly formed meteorite." You’d think, "Wait a minute, a meteorite? That’s not in any normal cake recipe!" And similarly, a nitrogen atom isn't in the fundamental recipe for a monosaccharide.
So, to recap, monosaccharides are characterized by:
- Being the simplest form of sugar.
- Possessing a carbonyl group (either aldehyde or ketone).
- Having multiple hydroxyl groups.
- Having a specific number of carbon atoms (triose, tetrose, pentose, hexose, etc.).
They are not characterized by:
- The presence of nitrogen atoms within their basic structure.
Next time you're enjoying something sweet, whether it's a piece of fruit or a slice of that mythical 1950s cake, you can appreciate the fundamental building blocks that make it all possible. And you’ll know, with scientific certainty, that those simple sugars are defined by their elegant simplicity, not by the addition of cosmic dust or, in this case, nitrogen.
It’s pretty cool to think about, isn't it? The universe of chemistry, all boiled down to these tiny, fundamental units. And sometimes, the answer to a complex question is simply about identifying what doesn't belong. Just like finding that one ingredient in Grandma's recipe that feels a bit out of place.
