Mass Density Of Water In Kg M3: Complete Guide & Key Details
Ever found yourself staring at a glass of water, maybe wondering why it behaves the way it does? Or perhaps you've lugged a bucket of the stuff and thought, "Crikey, this is heavier than it looks!" Well, my friends, you've stumbled upon a little scientific secret that's as common as a rainy Tuesday: the mass density of water. Don't let the fancy term scare you; it's basically a fancy way of saying 'how much oomph is packed into a certain amount of water.' Think of it like the difference between a fluffy cloud and a brick – both are made of stuff, but one feels a whole lot more substantial, right?
In our quest for a complete guide to this watery wonder, we're going to dive deep (pun intended!) into the numbers, but we'll do it with a smile and maybe a few chuckles along the way. No need for lab coats or complex equations here. We're talking about the stuff that fills our oceans, our bathtubs, and yes, that very glass of water you might be sipping from right now.
The Nitty-Gritty: What is Mass Density Anyway?
Alright, let's break it down. Mass density is a property of matter. It tells us how much stuff (that's the mass, usually measured in kilograms, or kg) is crammed into a specific amount of space (that's the volume, usually measured in cubic meters, or m³). So, when we talk about the mass density of water, we're asking: "How many kilograms of water can we fit into a neat little box that's one meter long, one meter wide, and one meter high?"
Imagine you have a magical box, precisely one meter on each side. Now, fill that box to the brim with water. The weight of that water, in kilograms, is essentially its mass density. It's like measuring how much a specific size of Tupperware weighs when filled with your grandma's famous potato salad. Some potato salads are lighter and fluffier, others are dense and packed with every delicious ingredient imaginable. Water, in its most common form, has a pretty consistent "recipe."
So, for water, the magic number we're often dealing with is around 1000 kilograms per cubic meter (1000 kg/m³). That's a pretty solid number, and it means that if you had that giant, meter-cubed box of water, it would weigh approximately 1000 kilograms. That's roughly the weight of a small car! Imagine trying to lift that box. You'd probably want a crane, or at least a really good team of friends with strong backs and a shared love of physics.
Water's Weighty Reputation: Why 1000 kg/m³?
Now, why 1000 kg/m³? It's a number that's super convenient for us humans because it's so close to a whole unit. It makes calculations a breeze. Think about it: if a cubic meter of water weighs 1000 kg, then half a cubic meter weighs about 500 kg, and so on. It’s like having a measuring tape where every foot marker is a whole number – much easier to eyeball than dealing with fractions of feet constantly.
This figure of 1000 kg/m³ is often considered the standard density of pure water. But hold on, before you get too attached to that number, it’s important to know that water isn't always exactly 1000 kg/m³. It's like your favorite recipe – if you add a pinch more salt or use slightly different sized eggs, the final cookie might be ever so slightly different. Water is a bit like that.
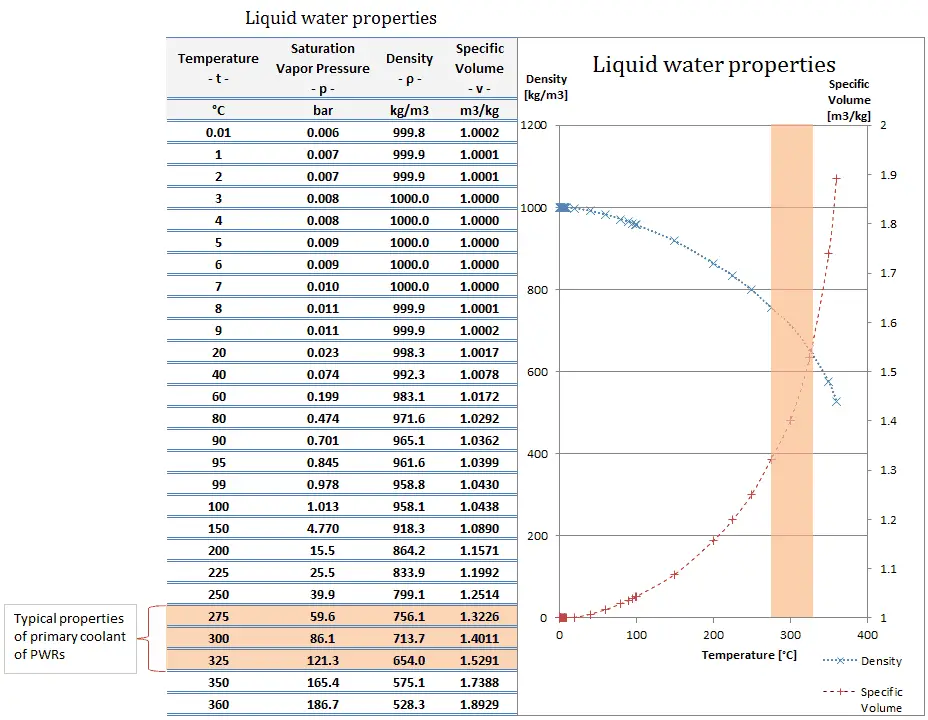
The density of water can actually change based on a couple of key factors, and these are the "key details" that make this whole thing even more interesting. We're talking about temperature and pressure. Sounds a bit technical, but trust me, you’ve experienced these effects without even realizing it!
Temperature: The Great Water Expander (and Contracter!)
Let's talk about temperature. When you heat things up, they generally like to spread out. Think about popcorn popping. The kernels expand, right? Water does something similar. As water gets warmer, its molecules get more energetic and start bouncing around more, taking up a bit more space. This means that a certain amount of warm water will actually have a slightly lower density than the same amount of cold water. It’s like a crowded party – when the music starts and everyone starts dancing, they take up more room!
The coolest (or warmest!) part about water's density and temperature is that its maximum density actually occurs at around 4 degrees Celsius (4°C). Yep, you read that right. As water cools down from, say, room temperature towards freezing, it gets denser. But then, something magical (and super important for aquatic life) happens. As it gets closer to freezing (0°C), it actually starts to become less dense again! This is why ice floats. If ice were denser than water, our lakes and oceans would freeze from the bottom up, which would be a rather messy situation for fish and pretty much anything else living in them. Talk about a chilly predicament!
So, while 1000 kg/m³ is our handy benchmark, remember that at room temperature (say, 20°C), water is actually a touch less dense, closer to 998 kg/m³. And when it’s really cold, just above freezing, it’s also a little less dense than its maximum at 4°C. It’s water’s way of keeping us on our toes!
Everyday Encounters with Water's Density
You’ve probably encountered this density change without even thinking about it. Ever noticed how hot water seems to rise more quickly than cold water? That’s because the hotter water is less dense, so it naturally floats up. Think of it like bubbles in a hot drink – they’re less dense than the surrounding liquid, so they make a beeline for the surface. It's the same principle, just on a grander scale.
Or consider your swimming pool. On a hot summer day, the surface water might feel warmer and slightly less substantial, while the deeper water is cooler and feels more "solid" when you dive in. That’s water’s density at play, influenced by the sun’s warmth. It’s like a natural stratification system, a gentle reminder that even the seemingly simple things in life have their own intricate rules.
Pressure: The Squeezer of Water Molecules
Now, let’s talk about pressure. Imagine squeezing a sponge. When you apply pressure, you're forcing the stuff inside to pack closer together. Water is similar, though it’s a lot harder to squeeze than a sponge. As the pressure increases, the water molecules get pushed closer together, making the water slightly denser.
However, for most of the pressures we encounter in everyday life, the effect of pressure on water density is pretty minor. We’re talking about the difference between a leisurely stroll and a brisk jog in terms of how much it affects density. The pressure at the bottom of your bathtub isn’t going to make the water noticeably denser. You’d need the immense pressure of the deep ocean or some seriously high-tech laboratory equipment to see a significant change due to pressure alone.
Think of it this way: imagine trying to compress a giant Jell-O mold. It’s going to resist a lot more than a cushion. Water is quite incompressible compared to gases, which is why its density doesn't change dramatically with normal pressure fluctuations. So, while pressure does play a role, especially in extreme environments, for your daily dose of water science, temperature is usually the bigger player.

The Weight of Water: Real-World Calculations
So, why should you care about the mass density of water in kg/m³? Well, it’s surprisingly useful! Engineers use it to design dams, bridges, and pipelines. They need to know how much force all that water will exert. Farmers use it to calculate irrigation needs. And even for us home cooks, understanding density can be handy for things like measuring ingredients accurately.
For instance, if a recipe calls for "1 liter of water," and you know that 1 liter is equal to 0.001 cubic meters (since 1 m³ = 1000 liters), and water's density is roughly 1000 kg/m³, then you can easily calculate that 1 liter of water weighs approximately 1 kilogram. That’s a neat trick, right? No need for a scale if you're just measuring water!
It’s also why a full bathtub feels so much heavier than an empty one. The sheer volume of water, combined with its density, adds up to a significant weight. Imagine trying to carry that tub of water down the stairs – it would be a monumental (and probably disastrous) undertaking! That’s the power of density making itself known in the most tangible ways.
Beyond Pure Water: What About Saltwater?
We’ve been talking about pure water, but what about the vast oceans? That’s saltwater, and it’s a little different. When you dissolve salt (or other minerals) into water, you’re adding more stuff without significantly increasing the volume. This makes saltwater denser than pure water. Think of it like adding marbles to a glass of water; the water level rises a bit, but you've added a lot of extra weight.
.jpg)
This is why you float more easily in the ocean than in a freshwater lake or pool. Your body has a certain density, and if the surrounding water is denser, it provides more buoyancy. It's like the ocean is giving you an extra little "lift" to keep you afloat. That’s the magic of dissolved substances!
So, the density of seawater is typically higher than 1000 kg/m³, often around 1025 kg/m³ or even more, depending on the salinity and temperature. It’s another fascinating variation on our watery theme.
The Takeaway: Water's Wonderful Density
So, there you have it! The mass density of water in kg/m³ isn't just a dry number for textbooks. It's a concept that touches our everyday lives, from the way ice floats to why the ocean feels different from your local swimming pool. It’s a fundamental property of the substance that makes our planet so unique and life-sustaining.
Remember, the standard figure of 1000 kg/m³ is our trusty guide, representing the approximate density of pure water. But keep in mind that temperature is the real diva here, causing water to expand and contract, thus subtly altering its density. Pressure plays a supporting role, becoming more significant in extreme conditions.
The next time you fill up a glass, take a bath, or gaze out at a body of water, take a moment to appreciate the invisible force of its mass density. It’s a silent, constant, and utterly essential part of our world, as familiar and comforting as a cool drink on a hot day. And who knows, maybe you’ll even crack a smile thinking about that imaginary, car-sized box of water!
