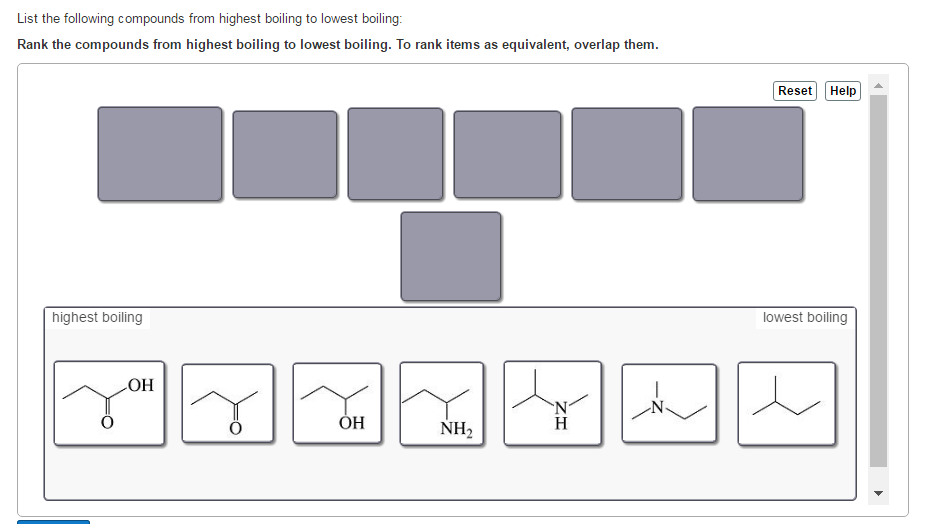
List The Following Compounds From Highest Boiling To Lowest Boiling
Ever find yourself staring at a list of ingredients, or maybe a scientific formula, and wonder what makes one thing behave so differently from another? It’s a bit like organizing your spice rack, but with molecules! We're talking about the fascinating world of chemical compounds and, more specifically, their boiling points. Sure, it might sound a little technical, but understanding boiling points can unlock some surprisingly practical insights into our everyday lives, from how our coffee stays liquid to why certain cleaning products work better than others.
So, why do people get a kick out of ranking these compounds? It’s all about understanding order and predictability in the often chaotic world of matter. For chemists, it's fundamental to their work, helping them separate and purify substances. But for us regular folks, it’s about appreciating the subtle, yet crucial, differences that make the world around us function. Think about it: the ability to boil water for your morning tea relies on water's specific boiling point. Cooking food, sterilizing equipment, even the way gasoline vaporizes in your car engine – all are dictated by these molecular characteristics.
The purpose it serves is to help us comprehend how different substances behave under varying temperatures. Knowing which compound boils at a higher or lower temperature allows us to make informed decisions. For instance, when you're choosing a solvent for a DIY project, understanding its boiling point can prevent dangerous fumes or ensure it evaporates at a rate that’s suitable for your needs. In the kitchen, it helps us understand why some oils can be heated to higher temperatures for frying than others. It’s essentially a peek behind the curtain of everyday phenomena.
Let’s dive into a specific scenario: comparing the boiling points of a few common compounds. Imagine we have:

- Water (H₂O)
- Ethanol (C₂H₅OH)
- Methane (CH₄)
- Sodium Chloride (NaCl)
Now, let's arrange them from highest boiling point to lowest boiling point. This ranking is based on the strength of the forces holding their molecules together. Stronger forces mean you need more energy (heat) to break them apart and turn them into a gas. So, from highest to lowest, we have:
- Sodium Chloride (NaCl): This ionic compound has incredibly strong electrostatic attractions between its positive sodium ions and negative chloride ions. It boils at a whopping 1413°C! You won't be boiling table salt anytime soon in your kitchen!
- Water (H₂O): Water has significant hydrogen bonding between its molecules, a strong type of intermolecular force. Its boiling point is a familiar 100°C.
- Ethanol (C₂H₅OH): While ethanol also exhibits hydrogen bonding, it's generally weaker than in water due to its larger, non-polar hydrocarbon chain. It boils at around 78.37°C.
- Methane (CH₄): This is a simple hydrocarbon with only weak Van der Waals forces between its molecules. It boils at a very low -161.5°C, meaning it’s a gas at room temperature and even on a very cold day!
To enjoy this exploration more effectively, try to connect these concepts to things you see and use daily. Notice how different cooking oils behave when heated – that’s a hint at their boiling points. When you’re cleaning, consider the volatility of the cleaning agents. Understanding these molecular behaviors can make you a more informed consumer and a more curious observer of the world around you. It’s a simple list, but it reveals a lot about the invisible forces shaping our physical reality!
