Ionic Compounds Are Normally In Which State At Room Temperature

So, I was helping my niece with her science homework the other day, and we got onto the topic of… drumroll… chemical compounds! Exciting stuff, right? She’s eight, so her idea of exciting is usually glitter and unicorns, but even she perked up when we started talking about things that look like they belong in a lab. We were mixing stuff in little plastic cups (don’t worry, it was just baking soda and vinegar, the classic volcano experiment) and she asked me, “Why does the fizz go everywhere when we do this?”
And that got me thinking. Chemistry is everywhere, isn't it? From the fizzy eruption of a homemade volcano to the salt we sprinkle on our fries, it’s all about these tiny, invisible building blocks called atoms and how they hook up to form compounds. Some compounds are super chill, others are… well, a bit more dramatic. Today, we’re diving into a particularly common and fascinating group: ionic compounds. And the big question on everyone’s mind (or at least, it should be if you’re me and you’re thinking about homework with an eight-year-old) is: what state are these guys usually in when we’re just hanging out at room temperature?
The Salt of the Earth (Literally!)
Let’s start with the most famous ionic compound of them all: sodium chloride. Yep, that’s plain old table salt. You know, the stuff that makes your popcorn taste amazing and prevents your roads from turning into ice rinks in the winter. Think about salt. What form does it usually take? It’s that nice, crystalline solid, right? You can pour it, it feels gritty between your fingers (though maybe don't go tasting your science experiment unless you’re sure what it is!), and it definitely isn't gurgling like water or floating away like air.
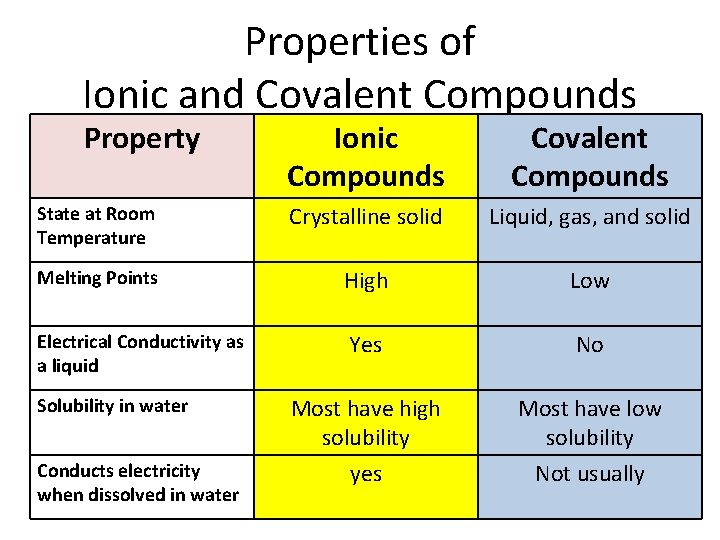
This is our first big clue. Ionic compounds, for the most part, are happy as solids at room temperature. And when I say room temperature, I mean that comfy range you're probably in right now, maybe between 20 and 25 degrees Celsius (that’s around 68 to 77 Fahrenheit for my non-metric friends). It’s the temperature where you’re not sweating buckets and you’re not reaching for a parka.
Why So Solid, Though?
Okay, so they’re solids. But why? This is where we have to get a little nerdy. Ionic compounds are formed when atoms get together and have a bit of a transfer of power. Imagine one atom with way too many electrons, practically begging to share. And another atom that’s just itching to take some off its hands. This is the basis of ionic bonding. One atom, typically a metal (think sodium, potassium, calcium), loses an electron to become a positively charged ion (a cation). The other atom, usually a non-metal (like chlorine, oxygen, or fluorine), gains that electron to become a negatively charged ion (an anion).
Now, here’s the crucial part: opposite charges attract. It’s like that classic saying about people, but for atoms! The positive cation is drawn to the negative anion like a magnet. And this attraction is super strong. We’re not talking about a gentle nudge; we’re talking about a powerful, electrostatic hug that holds these ions together.
These ions don't just form a little pair; they arrange themselves in a very organized, repeating pattern called a crystal lattice. Imagine a perfectly stacked pile of LEGO bricks, but instead of plastic, it’s ions, and instead of just being stacked, they’re held together by incredibly strong electrical forces. This orderly, tightly packed structure is what gives ionic compounds their characteristic solid state at room temperature.
Think about it: to break this lattice structure, to get these ions to move around freely like they do in a liquid or a gas, you need a whole lot of energy. You need to overcome those powerful attractions. And room temperature just doesn’t cut it for most ionic compounds.
Melting and Boiling: A Lot of Work!
This brings us to their melting and boiling points. Because the forces holding the ions together in the crystal lattice are so strong, ionic compounds typically have very high melting points and high boiling points. This is a key difference between ionic compounds and, say, water (H₂O). Water is a molecular compound, and the forces between water molecules are much weaker than the ionic bonds in a salt crystal. That's why water melts at 0°C and boils at 100°C – pretty chill temperatures compared to many ionic compounds.
For example, sodium chloride (your table salt) melts at a scorching 801°C and boils at a whopping 1413°C! Can you even imagine? That's hotter than the surface of some volcanoes! Clearly, we're not going to find salt spontaneously turning into a puddle of liquid just because it's a warm summer day. It needs some serious heat to get those ions moving.
Other common ionic compounds are even more stubborn. Calcium oxide (used in cement) melts around 2572°C. That’s hotter than a blast furnace! So, yeah, solid is definitely their default setting at anything remotely resembling normal temperatures.

Are There Any Exceptions? (Because Science Loves an Exception)
Now, you know me, I can't just give you a blanket statement and call it a day. Science is all about nuance, right? Are there any ionic compounds that are not solids at room temperature? Technically, yes, but they’re usually pretty extreme cases or involve something a bit unusual.
For instance, some ionic compounds with very large, complex ions or very weak attractions between them might have lower melting points. However, these are not the typical, everyday ionic compounds you’d encounter. Most of the ones you’ll learn about in introductory chemistry – like the salts of alkali metals (lithium, sodium, potassium) and halogens (fluorine, chlorine, bromine) – are definitely solids.
Another thing to consider is what happens when you put ionic compounds in water. This is where they get interesting! When you dissolve an ionic compound like salt in water, the water molecules (which are polar) can surround and pull apart the individual ions. This means the compound is still made of ions, but they are now free to move around in the water. This is why saltwater conducts electricity! The mobile ions act as charge carriers. So, while the compound itself is a solid, its dissolved state is quite different.
But the question was about their state at room temperature in their pure form, not dissolved. And in that context, the answer is overwhelmingly solid.

Crystal Clear Properties
The crystalline structure of ionic compounds also leads to other interesting properties. Have you ever seen salt crystals up close? They often have a very defined shape. Ionic compounds tend to be hard but also brittle. This might sound contradictory, but think about it: the strong ionic bonds make them hard to scratch or deform. However, if you hit them hard enough, you can cause the layers of ions to shift. When this happens, ions with the same charge end up next to each other. And remember what happens when like charges get close? They repel! This repulsion can cause the crystal to shatter.
So, while they are tough, they’re also fragile, much like a perfectly constructed gingerbread house that looks amazing but would crumble if you sneezed too hard. It’s all about that organized lattice and the powerful forces within it.
Another fascinating property tied to their ionic nature is that they don't conduct electricity in their solid state. Since the ions are locked in place within the crystal lattice, they can’t move to carry an electrical current. It’s only when they are melted or dissolved in water that they become good conductors. This is a really important distinction and often tested in exams, so keep it in your mental chemistry notebook!
Everyday Encounters with Ionic Solids
Let’s bring it back to the real world. Where else do we see these ionic solids besides the salt shaker? Loads of places!

- Minerals and Rocks: Many rocks and minerals are made of ionic compounds. Think of things like quartz (silicon dioxide, SiO₂ – technically a covalent network solid but shares some characteristics of hardness and high melting point due to strong bonding), or even common rocks like limestone (calcium carbonate, CaCO₃). These are all solid structures formed by ions held together tightly.
- Fertilizers: Compounds like potassium nitrate (KNO₃) are used as fertilizers to give plants essential nutrients. They are typically supplied as granular solids.
- Soaps and Detergents: While more complex, many soaps and detergents have ionic components that help them clean. These components are often solids at room temperature before they dissolve.
- Ceramics and Glass: Many ceramics and glasses are based on oxides, which are often ionic or have significant ionic character. These are manufactured and used as solids.
It's pretty amazing how these simple arrangements of charged atoms can create such diverse and essential materials that make up our world. From the food on our plates to the ground beneath our feet, ionic compounds are silently playing their part, usually as a stable, crystalline solid.
The Takeaway
So, to wrap this up in a neat little bow (or maybe a tiny, organized crystal structure), the next time you reach for the salt, or think about minerals in the earth, remember that ionic compounds are normally solids at room temperature. This is due to the incredibly strong electrostatic forces between oppositely charged ions that arrange themselves into a rigid crystal lattice.
It’s this inherent structural integrity that keeps them firm, unmoving, and decidedly solid until you’re ready to apply some serious heat or dissolve them in a good solvent. It’s their default setting, their comfy place, their raison d'être in the world of chemistry.
And just a little tip from your friendly neighborhood science blogger: when you’re dealing with ionic compounds, always think strong attraction, crystal lattice, and high melting/boiling points. That’s the winning trifecta for understanding their state at room temperature. Now go forth and impress your friends with your newfound ionic wisdom!
