Interpreting The Angular Probability Distribution Of An Orbital
Alright, so imagine you're at a bustling café, right? The espresso machine is hissing like a dragon that's just stubbed its toe, and someone's practicing their opera singing on the street outside. And I lean in, cupping my coffee, and I say, "You know, we need to talk about the angular probability distribution of an orbital." I guarantee you, that's how you get the barista to spill your latte. But stick with me, because it’s actually way cooler – and way less sticky – than it sounds.
First off, what in the name of all that is holy is an "orbital"? Think of it like this: atoms, these tiny building blocks of everything, have electrons zipping around the nucleus. Now, you might picture them like tiny planets orbiting a sun, all neat and tidy. Nope! Nature, being the mischievous prankster it is, decided that's too simple. Electrons are more like… hyperactive toddlers at a rave. You know where they are, but you have no idea where they're going next. So, an orbital isn't a path; it’s a 3D cloud of probability. It’s where you’re most likely to find that little electron menace.
And the angular probability distribution? This is where it gets spicy. Forget the planets again. We're talking about the shape of that probability cloud. It's like trying to describe a cloud's shape on a really windy day. Is it a fluffy bunny? A grumpy old man? A suspiciously cloud-shaped potato? The angular part tells us about the directions the electron is likely to be found in, relative to the nucleus. It's the difference between a perfectly round donut and a… well, a really weirdly shaped donut.
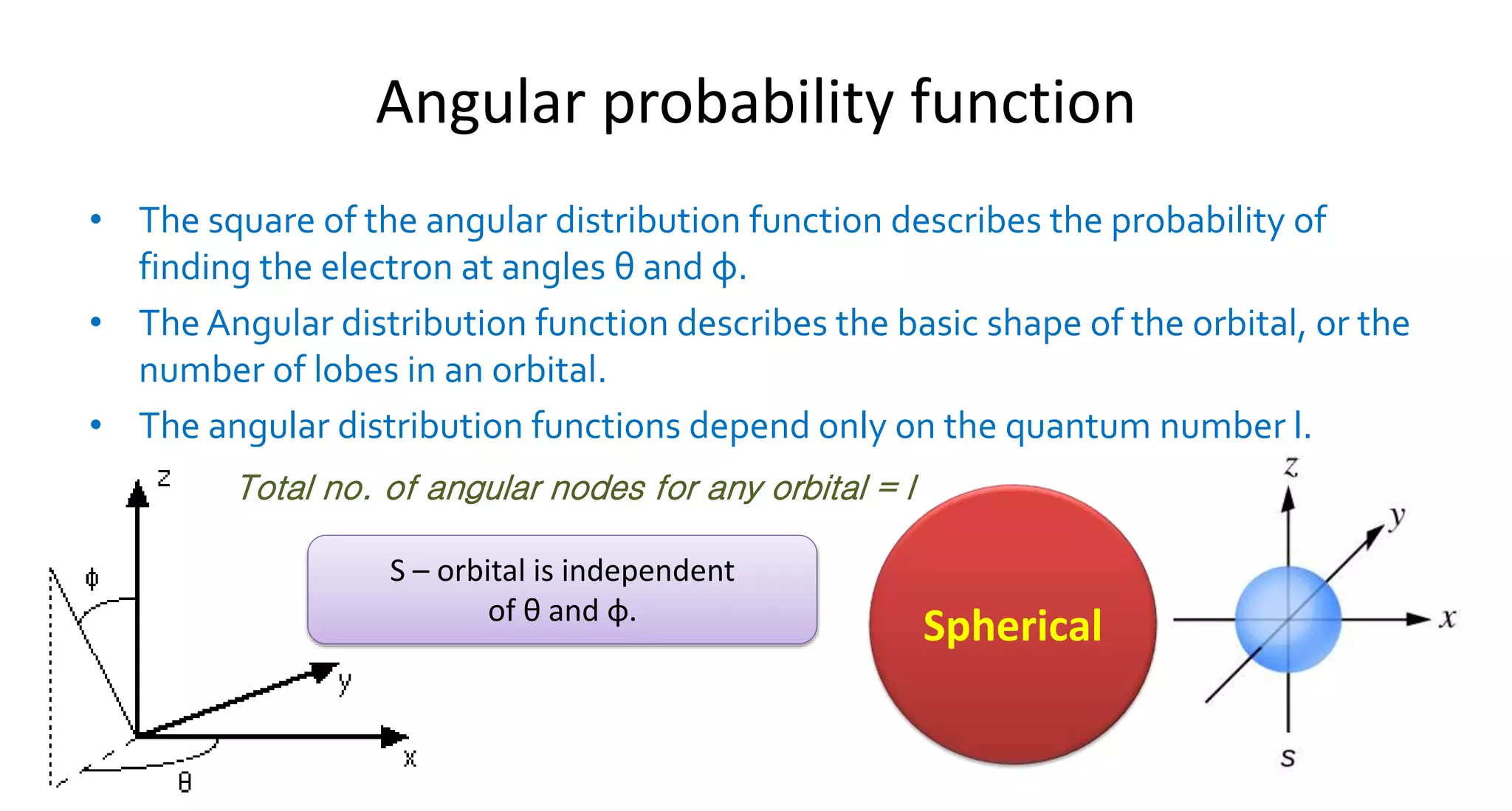
Let’s break down the usual suspects. You’ve got your s orbitals. These are the divas of the atom. They're perfectly spherical. Imagine a perfectly toasted marshmallow – that's an s orbital. No matter which direction you look from the nucleus, the chance of finding the electron is the same. It’s the introvert of the electron world: it just wants to be equally distant from everyone. Boring, perhaps, but reliable. Like that friend who always brings the same dish to the potluck, and it’s always amazing.
Then we get to the p orbitals. Oh, the p orbitals. These are where things start to get interesting. They look like dumbbells, or maybe a really enthusiastic infinity symbol. They have two lobes, with a little node – a place where the probability of finding the electron is zero – right in the middle, where the nucleus is chilling. This means the electron is never found right next to the nucleus in a p orbital. It’s like saying, "I love this party, but I’m definitely not talking to the host."
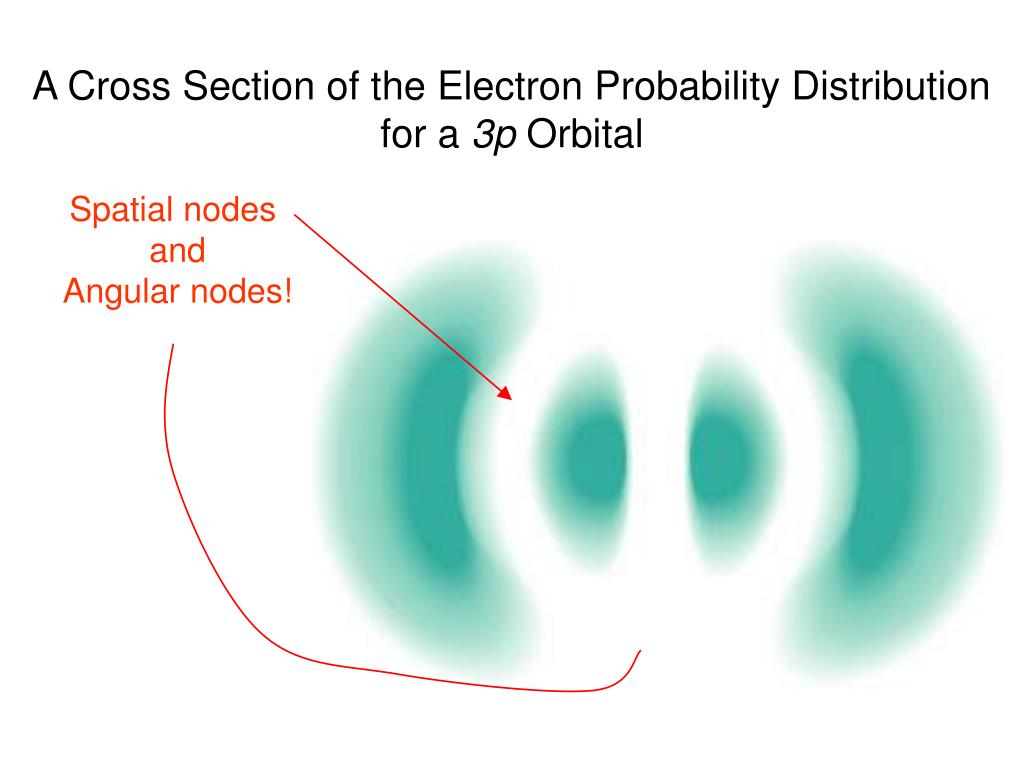
And here’s the kicker: there are three p orbitals! They’re usually called px, py, and pz. Why? Because they point along the x, y, and z axes. Imagine you’re drawing on a whiteboard. One p orbital is like drawing a line straight up and down. Another is a line left and right. The third is… well, it’s going into the third dimension, like it’s trying to escape the flat whiteboard and into our coffee-fueled reality. It’s the atom showing off its 3D skills. So, you have three dumbbell-shaped clouds, all centered on the nucleus, each pointing in a different direction. It’s like the atom has three mini-arms reaching out into space.
Now, the angular probability distribution for a p orbital is fascinating. If you were to plot it, it’s not a simple sphere. It’s got these two big bulges. The shape tells you that the electron is much more likely to be found in those two lobes than, say, directly between them. Think of it as a shy electron that prefers to hang out at the ends of its personal bubble, avoiding the crowded middle.
But wait, there’s more! We also have d orbitals. These guys are the true artists of the atomic world. They start getting a bit… exotic. Most d orbitals look like clovers, with four lobes. Yes, you heard that right. Four distinct regions of high probability, all sticking out from the nucleus like a hyperactive, four-leafed clover that’s been struck by lightning. It’s enough to make a mathematician weep with joy, or possibly terror.
There are five d orbitals, each with its own peculiar shape. One looks like a donut with two extra lobes, like a pac-man that’s gone rogue and sprouted extra cheeks. Another looks like two dumbbells crossed at the nucleus, which, again, is a zero-probability zone. The d orbitals are the rebels, the avant-garde artists of the electron cloud world. They’re not content with simple shapes; they want to push the boundaries of atomic geometry. They’re basically saying, "Spheres are for amateurs. We do art."
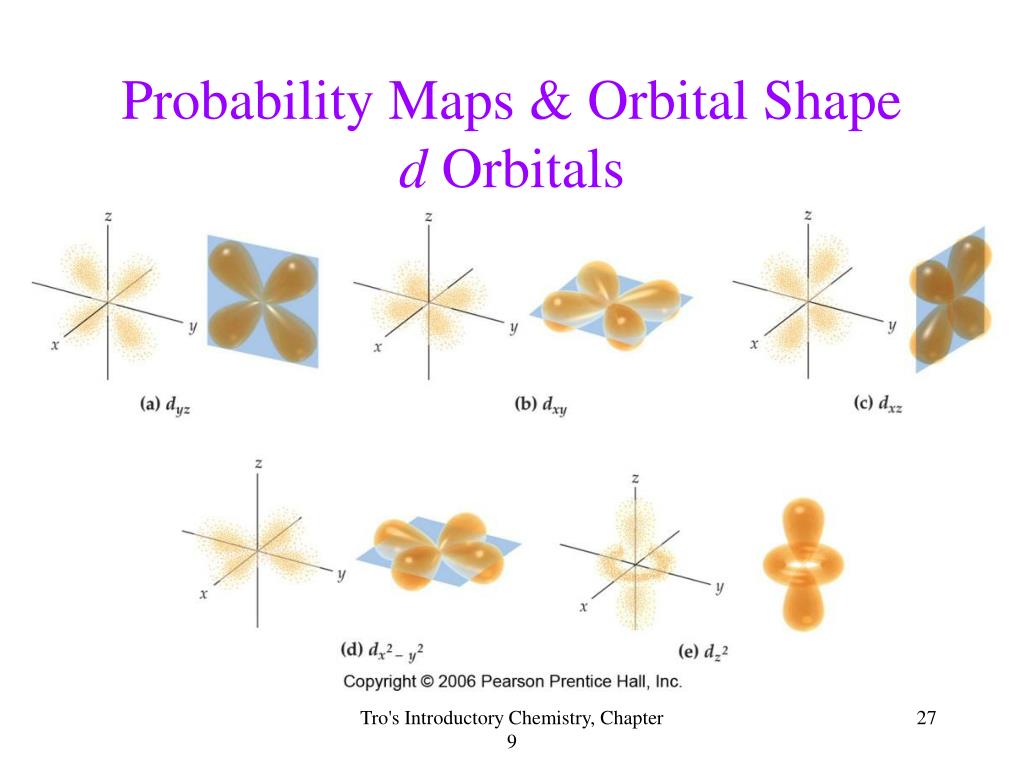
The angular probability distribution for a d orbital is where you see these complex, multi-lobed structures. It’s a visual representation of how the electron's wave function changes with direction. Don't worry about the "wave function" bit unless you brought a calculator and a strong constitution. Just know it's the mathematical description of the electron's behavior. The distribution is basically a fancy contour map showing you the hot spots – the places where finding the electron is practically guaranteed.
Why should you care about this anatomical weirdness of atoms? Because these shapes dictate how atoms interact! They determine how atoms bond together to form molecules. It's like understanding the handshake style of different people to know if they'll be best friends or awkwardly bump elbows. The shapes of orbitals dictate the chemistry we see all around us, from the deliciousness of that pastry you're eyeing to the very air you're breathing.
So, the next time you’re sipping your coffee, and you feel a bit contemplative, just remember the humble s orbital, the sassy p orbitals, and the flamboyant d orbitals. They’re not just abstract concepts for dusty textbooks; they’re the architectural blueprints of reality, dictating the very dance of atoms. And isn't that just the most wonderfully bizarre thing you've ever heard? It’s like the universe has its own secret rave, and we’re just starting to understand the DJ’s bizarre geometric beats.
