Indicate Whether Or Not Each Of The Structures Is Aromatic.

Okay, so imagine you've got a bunch of these little molecular structures chilling out. Some of them are super chill, some are a bit jumpy. We're gonna see which ones are the real deal, the ones with that special je ne sais quoi.
It's like trying to figure out if your friend is truly "extra" or just likes a good drama. We're talking about aromaticity, folks! And no, it has nothing to do with your fancy scented candles. Unless, of course, your scented candles are also tiny organic molecules. Which, come to think of it, is pretty cool.
So, let's dive into this world of rings and electrons. It's a bit like a molecular party, and we're the bouncers, deciding who gets in. Some structures are just too plain, too ordinary. They don't have the right vibe.
The Usual Suspects
First up, we've got good old Benzene. This guy is like the king of the aromatic castle. He's got that perfect ring, those special electrons that just love to dance in unison. He's the definition of aromatic. No arguments here.
Benzene: "Yep, I'm the OG aromatic. Deal with it."
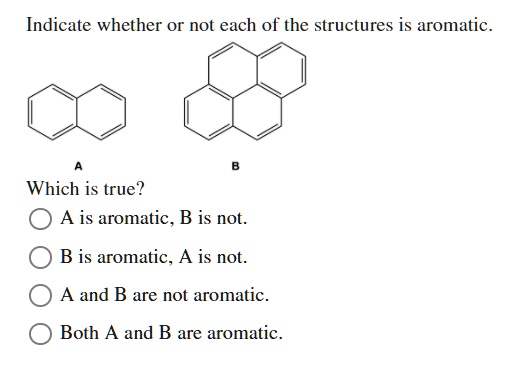
Then there's Naphthalene. This is like benzene's cooler, slightly more complicated cousin. He's got two rings fused together. Still totally aromatic, still rocking the vibe. He's just got more space to party.
And don't forget Anthracene and Phenanthrene. These guys are like the extended family of benzene. More rings, more electrons doing their thing. They're all part of the aromatic club, no question.
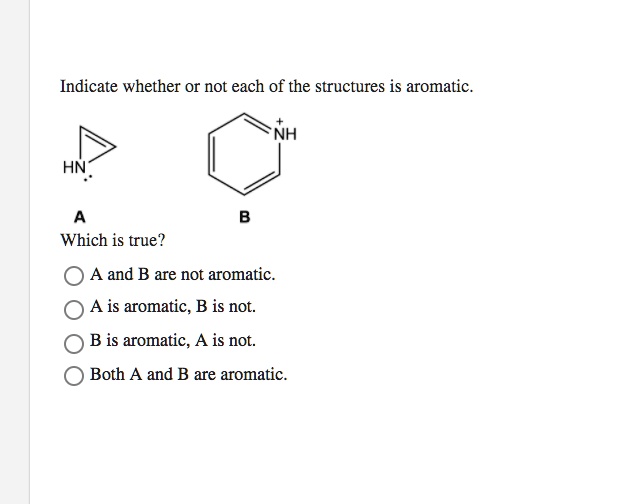
The Wannabes
Now, we start seeing some structures that look like they should be aromatic. They've got the rings, they seem to have the right number of atoms. But... something's missing.
Take Cyclooctatetraene. This one's a bit of a drama queen. It’s got eight atoms in a ring, and you might think, "Ooh, more the merrier!" But nope. This ring is all about twisting and turning to avoid that aromatic destiny. It's like it's actively anti-aromatic. Kind of a rebel, I guess.
Cyclooctatetraene: "Aromatic? Pfft. Too much commitment. I prefer to be... flexible."
Then we have structures that are so close, it's almost sad. They've got most of the requirements, but then one little thing is off. Maybe they have too many electrons, or not enough. It's like being one ingredient short of a perfect cake.
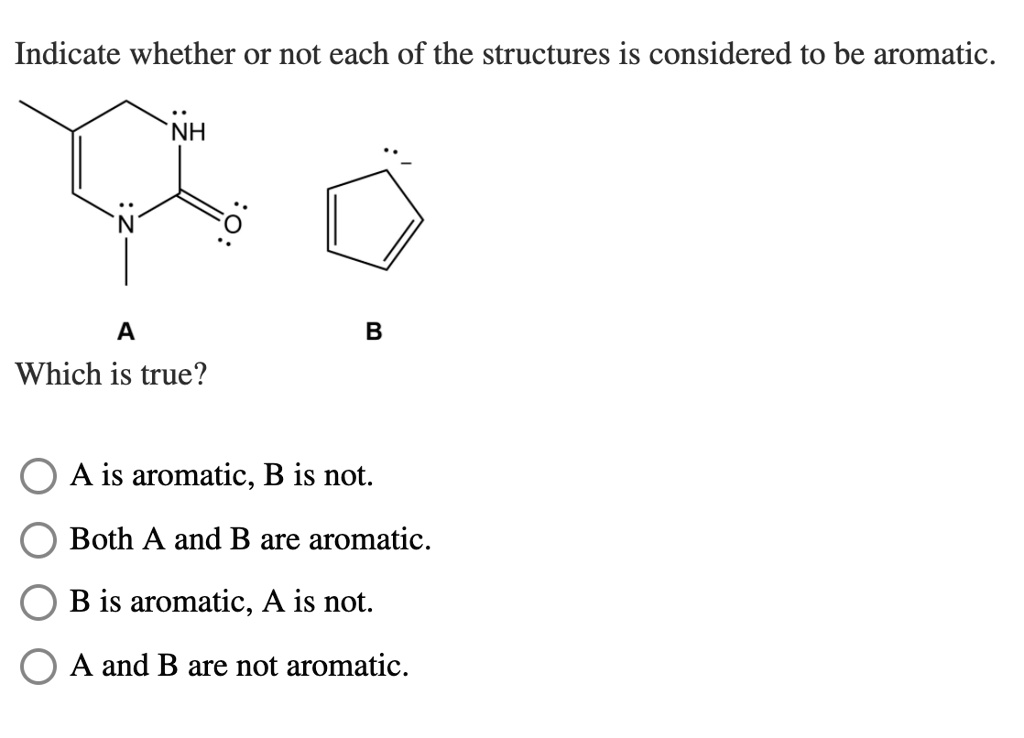
The Special Cases
We also have these tricky ones, like Pyrrole and Furan. They're five-membered rings, and they've got a heteroatom (like nitrogen or oxygen) thrown in. These guys can be aromatic, but it depends on how they're behaving. Are they sharing their electrons nicely in the ring? Or are they keeping some for themselves?
It's like asking if your roommate is really cleaning their share of the dishes. You have to check the evidence! If those extra electrons are joining the ring dance, then yes, they get the aromatic stamp of approval.
But then there’s Pyrazole and Imidazole. These are similar, but with an extra heteroatom. They're a bit more complex, like a puzzle. They can be aromatic, but you really have to look at their electron configuration. It's not always a straightforward yes or no.
The Non-Believers
And let's not forget the structures that are just... not feeling it. They're perfectly fine molecules, doing their own thing, but aromaticity just isn't in their DNA. Think of simple saturated rings, like Cyclohexane. This is the ultimate chill molecule. It's got no desire for electron delocalization or rigid planarity. It's happy being its flexible, non-aromatic self.
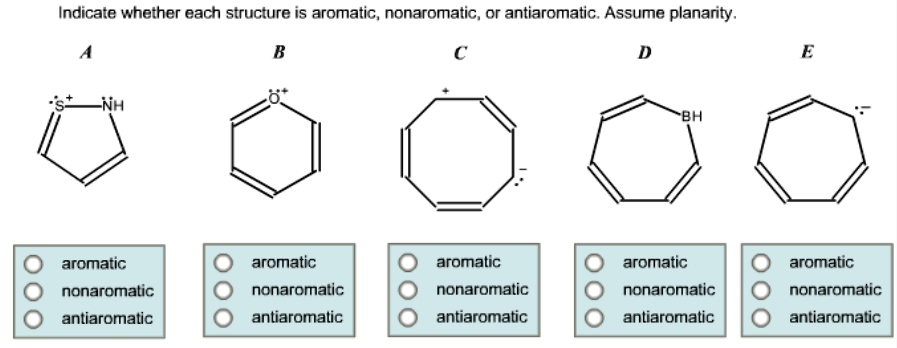
Cyclohexane: "Aromatic? What's that? Sounds like a lot of effort. I'm good."
Or even something like Cyclopentadiene when it's not in its deprotonated form. It’s got a double bond and a ring, but it's not quite there. It's got an extra electron hanging around that doesn't want to join the aromatic party. It’s almost there, but not quite. It’s the friend who shows up to the party without the required dress code.
The Oddballs
Sometimes, you get these structures that are just plain weird. They might have charges, or they might have atoms with unpaired electrons. These are the molecules that make you scratch your head and go, "Wait, what is happening here?"
Consider the Tropylium cation. This guy is a seven-membered ring with a positive charge. It’s got that perfect number of pi electrons, and it's planar. It's like a surprise guest at the aromatic party who turns out to be the life of it! Totally aromatic, despite the charge.
On the flip side, there’s the Cyclopentadienyl anion. This one is the charged counterpart to our previous cyclopentadiene friend. Now, with that extra electron, it becomes beautifully planar and has the right number of pi electrons. It's like it finally found its groove. Another aromatic champion!
The Verdict
So, to sum it up, being aromatic is all about a few key things. You need a ring, and that ring needs to be planar (flat). Then, the real magic happens with those pi electrons. They have to be able to move around freely within the ring, like a well-rehearsed dance troupe.
And for the icing on the cake, you need a specific number of these pi electrons. It's like a secret handshake: 4n+2. So, 2, 6, 10, 14… that’s the magic ticket. If you’ve got that, and the other conditions are met, then congratulations, you're officially aromatic!
It's a quirky set of rules, I know. And sometimes, even the chemists get it wrong at first glance. But that's the fun of it, right? Unraveling these molecular mysteries. So next time you see a ring structure, give it the sniff test. Does it have that aromatic sparkle? Or is it just another molecule in the crowd?
