In Which Species Does Phosphorus Have The Highest Oxidation Number
Get ready to have your mind blown by the humble element, Phosphorus! This amazing stuff is everywhere, from our bones to the sparks flying from a firework. But have you ever stopped to wonder just how powered up phosphorus can get? We're talking about its oxidation number, which is basically like its "energy level" or "how much it's willing to share its electrons."
Now, phosphorus is usually pretty chill, hanging out with an oxidation number of -3, like when it's cozying up in a phosphate. But sometimes, just sometimes, phosphorus can crank things up to eleven! It's like going from a lazy Sunday afternoon to a rock concert – a total transformation!
So, where does this supercharged phosphorus hang out? In which species does phosphorus achieve its highest oxidation number? Drumroll, please... it's in the world of the truly extreme!
The Reigning Champion of Phosphorous Power!
Hold onto your hats, folks, because the species that boasts phosphorus with its absolute highest oxidation number is none other than perchlorate compounds! Yes, you heard that right. We're talking about a state of pure, unadulterated, electrons-donated-to-the-max phosphorus.
Think of it this way: imagine phosphorus as a tiny, energetic kid who's just been given a mountain of candy. It's so excited, it's practically vibrating with energy and wants to give all its toys (electrons) away! That's kind of what's happening here.
In compounds like potassium perchlorate (KClO4) or even simpler forms like just the perchlorate ion (ClO4-), phosphorus is a positively charged superstar. Its oxidation number here reaches a magnificent +5! That's like going from being a beginner at video games to winning the world championship in a single leap!
Why is +5 so Special?
Phosphorus has five valence electrons, which are the ones it uses for bonding. To reach an oxidation number of +5, it means that phosphorus has pretty much given away all of those five electrons. It’s like it’s emptied its entire piggy bank to make friends!
This extreme giving of electrons makes phosphorus in perchlorates incredibly reactive. These compounds are often used in things that need a big burst of energy, like fireworks and rocket fuel. It’s the kind of power that makes you say, "Wow!"
Phosphorus at +5 is like a superhero who has just completed their ultimate power-up sequence. It’s ready to do amazing (and sometimes explosive!) things.
When phosphorus is at its +5 oxidation state, it's like it’s wearing a cape and flying through the chemical sky. It’s in its most oxidized, most electron-deficient state. This makes it a potent oxidizing agent, meaning it loves to take electrons from other things, making them more oxidized!
So, next time you see a dazzling firework display, remember the incredible power of phosphorus working overtime in those perchlorate compounds, reaching its peak at that amazing +5!
A Peek at Other Phosphorous Possibilities
While +5 is the undisputed champion, phosphorus loves to experiment with its oxidation numbers. It’s a chemical chameleon, always changing its colors (electron states) depending on who it’s hanging out with.
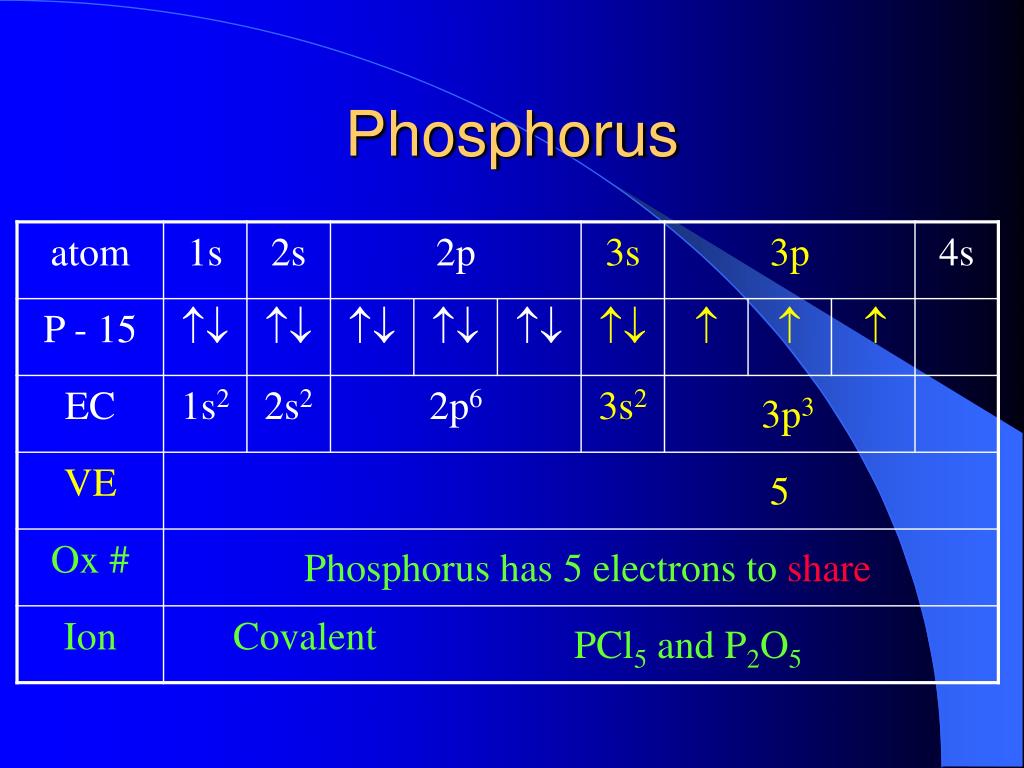
You'll often find phosphorus in the more common state of -3. This is when it’s in compounds like phosphine (PH3), a gas that’s a bit like the shy cousin of phosphorus. It’s holding onto its electrons quite tightly in this form.
Then there are the phosphates, like the ones that are super important for our bodies and plants. In phosphates (like PO43-), phosphorus is usually at a respectable +5 oxidation state too! So, even in the building blocks of life, phosphorus is showing off its energetic side.
But it doesn't stop there! Phosphorus can also be found at +1, +2, +3, and +4. Each of these states represents a different level of electron sharing, a unique chemical personality.
Think of it like a musical scale. -3 is like a low, rumbling bass note, while +5 is the soaring, high-pitched crescendo. All the numbers in between are the different melodies and harmonies that phosphorus can create.

The "In-Between" Wonders
Let's talk about some of the cool intermediate states. In compounds where phosphorus is bonded to both oxygen and other elements, things get really interesting.
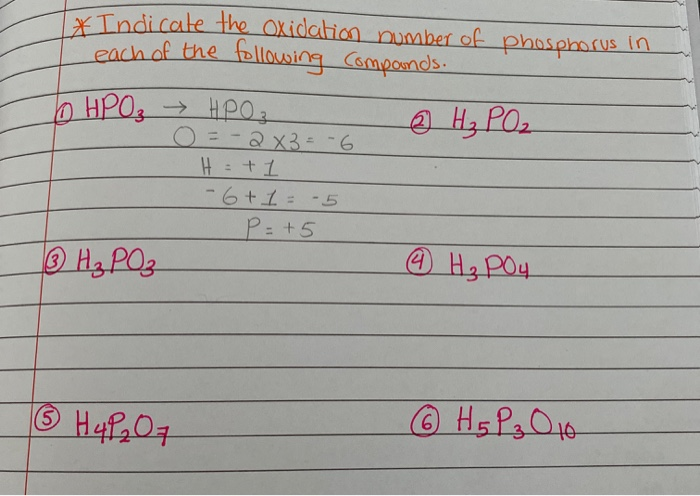
For example, in phosphorous acid (H3PO3), phosphorus is at a +3 oxidation state. This is a bit like phosphorus deciding to share most but not all of its electrons. It’s a balanced act, not going to the extreme of full surrender or complete hoarding.
And then there are compounds like hypophosphorous acid (H3PO2), where phosphorus shows up with a +1 oxidation number. This is like phosphorus thinking, "Okay, I'll share a little, but I'm still keeping a good chunk of my electrons for myself!" It’s a more reserved, yet still energetic, state.
These in-between states show the incredible versatility of phosphorus. It’s not just a one-trick pony; it’s a whole symphony orchestra of chemical possibilities!

The beauty of phosphorus is its adaptability. It can be a quiet observer or a boisterous performer, depending on the chemical company it keeps.
The fact that phosphorus can exist in so many different oxidation states is a testament to its electron configuration. It's like having a toolbox with a wide variety of tools, each perfect for a different job. And when it comes to reaching that ultimate energetic peak, perchlorates are the undisputed champions!
The Takeaway: Phosphorus is a Powerhouse!
So, to recap: while phosphorus is happy to be in many different "moods" with varying oxidation numbers, the undisputed king of its energetic expression, the species where phosphorus reaches its absolute highest oxidation number, is in the realm of perchlorates, hitting a dazzling +5!
It’s a reminder that even the most common elements can have hidden depths and extraordinary capabilities. Phosphorus, this often-overlooked element, can transform into a potent force when the conditions are just right.
Next time you witness a spectacular fireworks display or think about the powerful reactions that drive rockets, give a little nod to the amazing phosphorus and its incredible journey to the +5 oxidation state. It’s a true chemical wonder, showing us that even in the smallest of particles, there’s potential for immense power and dazzling displays!
The chemical world is full of these amazing stories, where elements take on different personas and achieve incredible feats. And in the grand opera of oxidation numbers, phosphorus in perchlorates is definitely hitting the highest, most powerful note!
