In Which Compound Does Chlorine Have The Highest Oxidation Number
Have you ever looked at a chemical formula and wondered what all those numbers meant? Specifically, those little superscript numbers that tell us about the "oxidation number" of an atom? It might sound a bit technical, but understanding oxidation numbers, especially for a common element like chlorine, can be surprisingly fun and even a little bit like solving a tiny puzzle! It's like unlocking a secret code within the building blocks of everything around us.
For beginners diving into chemistry, grasping oxidation numbers is a fundamental step. It helps make sense of how atoms interact and form bonds. For families doing at-home science experiments, it adds an extra layer of understanding to common household chemicals – think about bleach or even table salt! And for hobbyists, whether you're into gardening with fertilizers or even brewing your own kombucha, understanding these chemical properties can lead to more informed and successful projects. It’s all about making the invisible world of chemistry a little more visible and accessible.
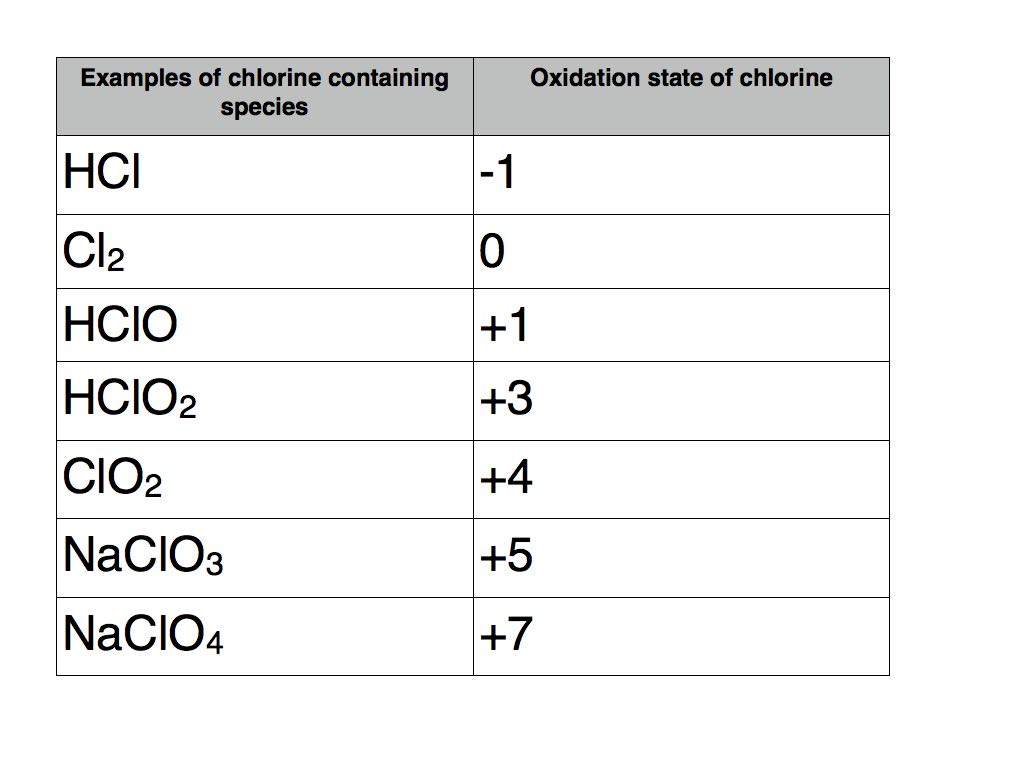
So, when it comes to chlorine, an element we encounter often (think swimming pools and disinfectants!), its oxidation number can vary quite a bit. Typically, we see chlorine with a -1 oxidation number, as in
You might also encounter chlorine with intermediate oxidation states. For example, in sodium hypochlorite (NaClO), the active ingredient in bleach, chlorine has an oxidation number of +1. Then there's sodium chlorite (NaClO₂) where it's +3, and sodium chlorate (NaClO₃) where it's +5. Each of these numbers tells us a different story about how the chlorine atom is behaving in that particular molecule. It’s a fantastic illustration of how elements can participate in chemistry in diverse ways.

Getting started with this concept is easier than you think! You don't need a fancy lab. Start by looking at the ingredients list on products around your home. Can you find any compounds containing chlorine? Next, try looking up the oxidation states of chlorine in those common compounds using a simple internet search. You'll be amazed at how quickly you can spot the patterns. Think of it as a treasure hunt for chemical facts!
Exploring oxidation numbers, especially the fascinating extremes that chlorine can reach, is a rewarding journey. It’s not just about memorizing facts; it's about appreciating the dynamic nature of chemical elements and the ingenious ways they combine. So next time you see a chemical formula, take a peek – you might just discover another fun puzzle waiting to be solved!
