In Photosynthesis Redox Reactions Ultimately Transfer Electrons From

Hey there, awesome explorer of the natural world! Ever wonder how plants, those silent, green superheroes, manage to whip up their own food? It’s not like they have tiny kitchens or anything, right? Well, it all boils down to a super cool process called photosynthesis. And the real magic behind it? It's a bit of a science-y term, but trust me, it's way less scary than it sounds: redox reactions.
Now, I know what you're thinking. "Redox? Is that some kind of ancient, dusty spell?" Nope! Think of it as a dance of electrons. Seriously, that's pretty much it. In the grand, leafy ballroom of a plant cell, electrons are doing a fantastic tango, and it's this very dance that fuels the entire operation. So, let's break down who’s leading and who’s following in this energetic electron shuffle. Basically, in photosynthesis, these electron transfers are the star of the show. They're the currency, the energy carriers, the whole shebang!
The Great Electron Hand-Off: Who's Giving What?
So, the big question is: where do these electrons come from? In the wild, wild world of photosynthesis, the ultimate source of these energetic little guys is none other than water. Yep, that H₂O stuff you chug when you're thirsty and that plants are constantly slurping up through their roots. Mind. Blown.
Think of it like this: water is like the plant's pantry, packed with all sorts of goodies. But the real treasure within that water, the stuff that gets this whole photosynthetic party started, is the electrons from the hydrogen atoms. The oxygen atoms in water are pretty chill, they’re like the supportive friends who are happy to let the hydrogen take center stage. The plant, in its infinite wisdom (or rather, its finely tuned biochemical machinery), knows exactly how to extract these precious electrons.
Water's Big Sacrifice
When we say water is the source, we're talking about a process called photolysis. Don't let the fancy name fool you. It literally means "splitting by light." So, when sunlight hits the plant, it provides the energy to break apart those water molecules. It's like the sunlight is a tiny, super-powered karate chop that splits H₂O into its components: oxygen (O₂), protons (H⁺), and, you guessed it, electrons (e⁻).
The oxygen? Well, it's kind of like the leftover packaging. The plant doesn't really need it for making its food, so it bravely releases it into the atmosphere. This is why plants are so awesome – they give us the very air we breathe! Thanks, plants! You’re literally breathing life into us while you’re busy making your own snacks. Talk about a win-win!

The protons (H⁺) are also important players in the later stages, kind of like the stagehands setting up the next act. But the real VIPs in this electron transfer saga are those liberated electrons. They’re now super energized, thanks to the sun’s rays, and ready for their next big adventure.
The Journey of an Energized Electron
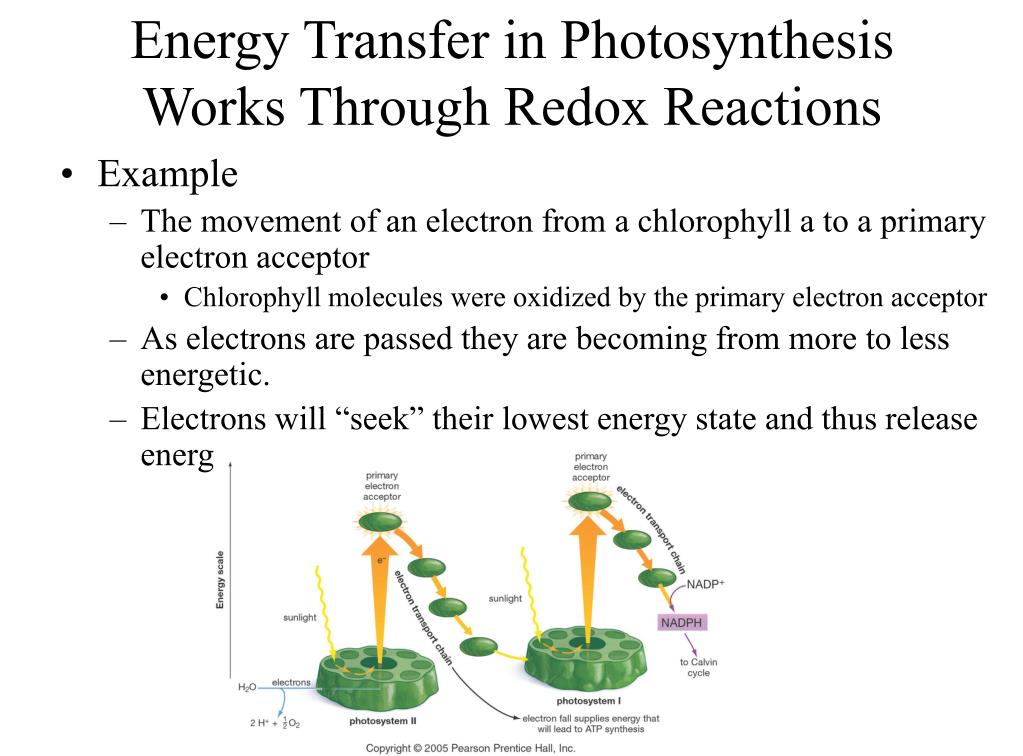
So, where do these high-energy electrons go after their water-splitting spa day? They embark on a thrilling rollercoaster ride through a series of protein complexes embedded within the plant cell's chloroplasts. These complexes are like a series of Olympic hurdles, and the electrons have to jump over each one, losing a bit of energy at each stage, but in a controlled way.
This chain of electron carriers is super important. It’s called the electron transport chain. Imagine a tiny, molecular assembly line. Electrons are passed from one molecule to the next, like a baton in a relay race. Each transfer releases a little bit of energy, and this energy is used to do some pretty cool work.
Powering Up the Proton Pump
One of the most crucial things this electron energy does is power a proton pump. Think of it as a tiny, biological water wheel that uses the energy from the electron transfer to shove protons (those H⁺ ions we mentioned earlier) from one side of a membrane to the other. This creates a concentration gradient, meaning there are way more protons on one side than the other. It's like building up pressure behind a dam.
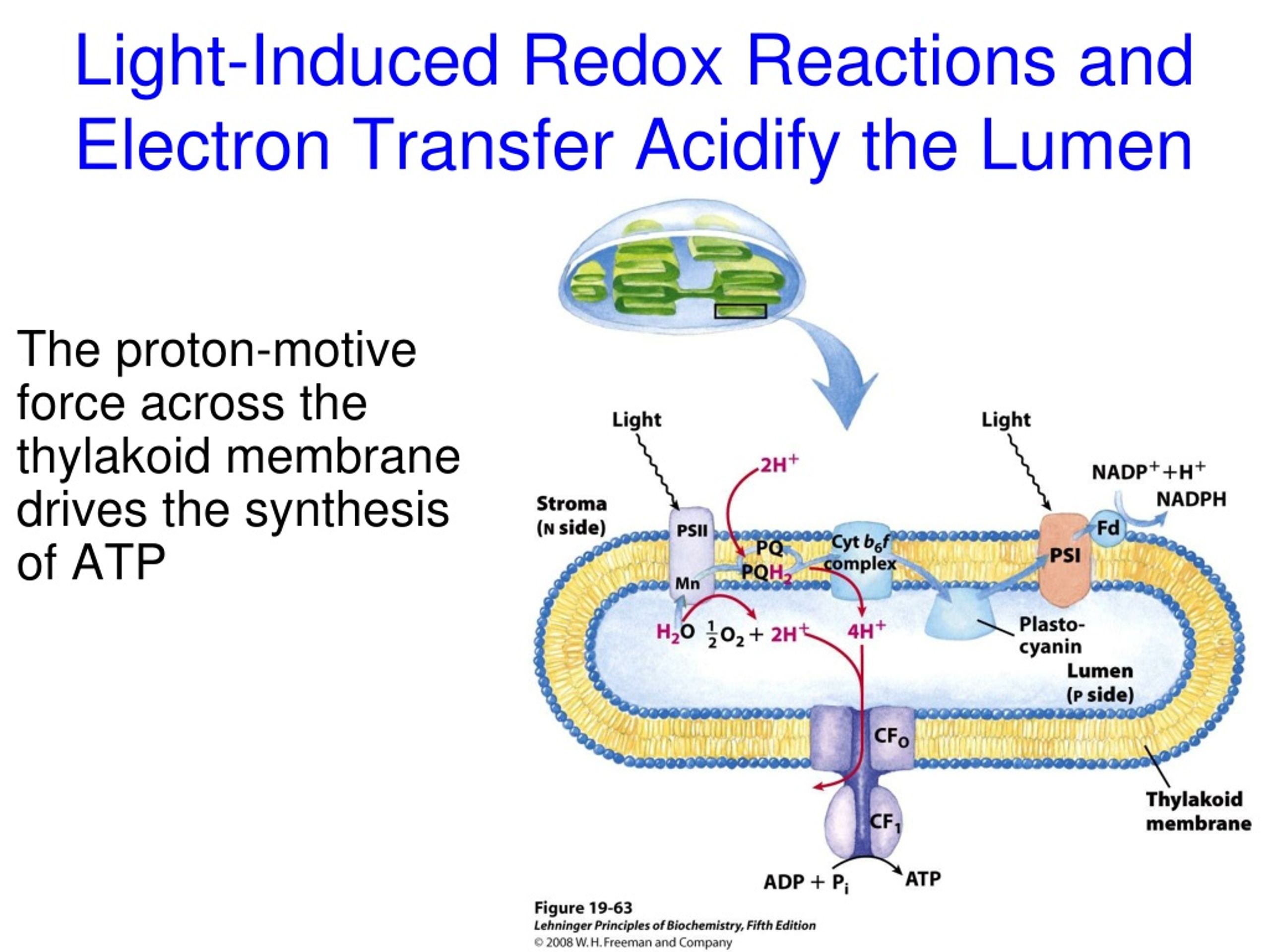
This buildup of protons is essential because it creates a form of stored energy. It’s like winding up a spring or pumping water uphill. This potential energy is then ready to be unleashed. It’s a classic example of how electrons, by moving and giving up their energy, can drive other processes. This whole electron transfer thing is not just for show; it's for doing!
The Final Destination: Capturing Energy
Eventually, after their exciting journey through the electron transport chain and powering the proton pump, these electrons have to go somewhere. They can't just wander off and start a new life as free radicals. Their final destination is to be handed off to a molecule that is quite literally the energy currency of the cell: NADP⁺.
NADP⁺, which is short for nicotinamide adenine dinucleotide phosphate (try saying that five times fast!), is like an electron acceptor. It’s hanging out, waiting for some electrons to donate to. When it finally receives a couple of electrons and a proton, it becomes NADPH. Think of NADPH as a fully charged battery, ready to deliver its energy and electrons to where they're needed next.
The Calvin Cycle: Building the Sweet Stuff
Now, this NADPH is a pretty important molecule because it’s one of the key players in the next phase of photosynthesis, known as the Calvin cycle. While the first part of photosynthesis (where water is split and electrons are transferred) is all about capturing light energy and converting it into chemical energy (in the form of ATP and NADPH), the Calvin cycle is all about using that captured energy to build sugars.
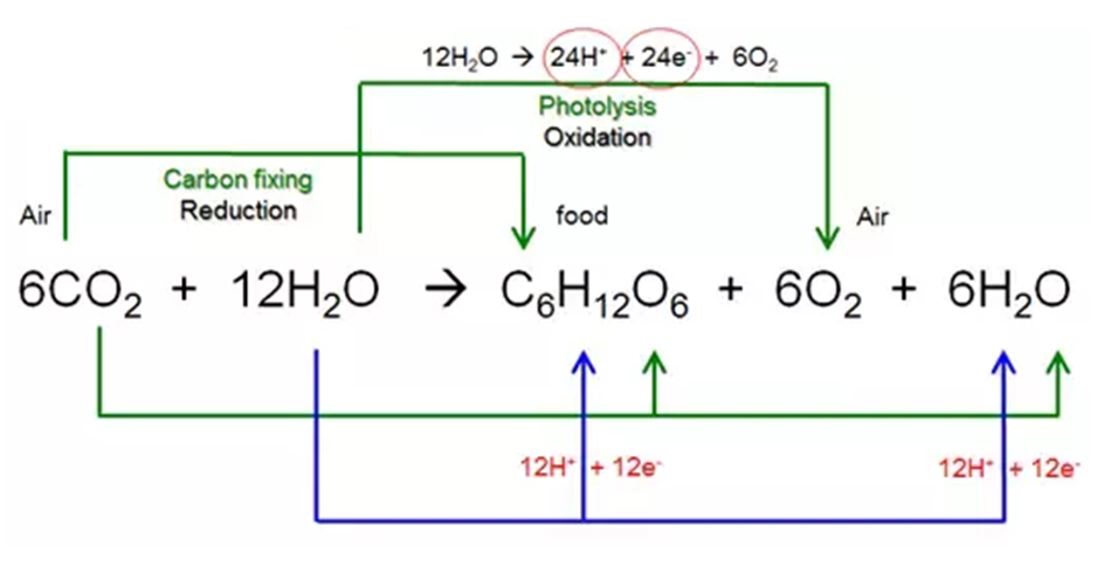
The electrons delivered by NADPH are used to reduce carbon dioxide molecules (CO₂), which the plant takes in from the air. This reduction process essentially means adding electrons and energy to CO₂, transforming it from a simple gas into more complex organic molecules, like glucose. So, the electrons that started their journey as part of a humble water molecule end up becoming the building blocks of the plant's food – the sugars that give it energy to grow, flower, and do all its amazing planty things!
The Redox Connection: A Summary
Let’s circle back to our main point: in photosynthesis, redox reactions ultimately transfer electrons from... drumroll please... water!
It's a beautiful cycle of giving and taking. Water gives up its electrons (oxidation), and other molecules in the photosynthetic machinery accept them (reduction). This continuous flow of electrons, powered by sunlight, is what drives the entire process. The energy captured from sunlight is essentially stored in the chemical bonds of those sugars, thanks to the electrons that started their journey in water.
So, the next time you see a lush green leaf, remember the incredible work happening within. It’s a tiny factory, running on sunlight and water, with electrons playing the starring role in a grand redox performance. It’s a testament to the elegance and efficiency of nature. From a simple molecule like water, to the energy-rich sugars that sustain life, it's all about that electron transfer!
A Little Joke to Finish
Why did the electron break up with the proton?
Because it felt too negative all the time!
(Okay, okay, maybe that one was a bit of a stretch, but you get the idea! Electrons can be a bit of a downer on their own, but they really shine when they're part of a team.)
Isn't it just incredible? From the tiniest water molecule to the magnificent trees that grace our planet, it’s all connected by this fundamental dance of electrons. Photosynthesis is more than just a biological process; it’s a daily miracle, a silent symphony of energy conversion that keeps our world alive and vibrant. So go forth, appreciate the green, and remember the epic journey of those electrons. They’re the unsung heroes, making our world a little brighter, one sip of water and one ray of sunshine at a time. Keep shining!
