Identifying Quantum Mechanics Errors In Electron Configurations

Hey there, ever feel like you’re juggling a million things at once? Like trying to remember where you put your keys, what you’re supposed to have for dinner, and if you remembered to water that one sad-looking plant? Well, imagine trying to keep track of tiny little things, like electrons, zipping around in atoms. That’s kind of what we’re talking about when we talk about electron configurations and how things can go a little… wonky.
Now, you might be thinking, “Electron configurations? Isn’t that some super-complicated science stuff?” And yeah, it can sound a bit intimidating, like trying to assemble IKEA furniture without the instructions. But stick with me, because understanding these little quirks in how electrons behave is actually pretty cool and, believe it or not, has a sneaky way of impacting our everyday lives. Think of it like this: even the tiniest wobble in a building’s foundation can eventually lead to a cracked wall, right? It’s the same principle, just on an atomic level.
So, what’s the big deal about getting an electron configuration wrong? Well, it’s like giving your GPS the wrong starting address. You might still get somewhere, but it’s probably not going to be where you actually wanted to go, and you’ll end up feeling a bit lost and confused. In the world of atoms, an incorrect electron configuration can lead to misunderstanding how elements behave, why they bond together the way they do, and ultimately, how materials are made.
Let’s get a little more specific. Atoms are made of a nucleus (that’s the core, like the pit in a peach) and electrons that whiz around it. These electrons aren’t just randomly flying around; they hang out in specific energy levels, kind of like floors in a building. And within those energy levels, they have even more specific spots called orbitals, which are like different rooms on each floor. Quantum mechanics, that mind-bending branch of physics, is the rulebook for these tiny electron tenants.
The Usual Suspects: Where Errors Creep In
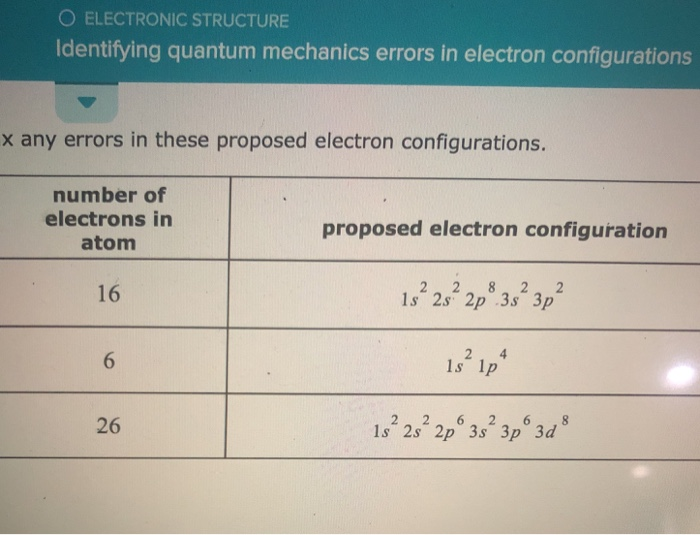
So, where do we, or rather, where do scientists (or even students learning this stuff!) go wrong with electron configurations? It’s usually when we forget the subtle rules of the quantum world. One of the most common slip-ups is related to something called the Aufbau principle. Think of this as the rule that says, “Fill up the lowest energy levels first, like filling a bucket from the bottom up.” It sounds simple enough, right? But sometimes, the energy levels can be really close in energy, and it’s not always as straightforward as just going in strict numerical order.
Imagine you have two small boxes and one big box, and you’re filling them with little marbles. You’d naturally fill the two small boxes before the big one, right? But what if the big box is almost the same size as the small ones, and the way the marbles settle can be a bit unpredictable? That’s a bit like how some electron orbitals can have very similar energy levels, and it can get tricky to know which one fills up exactly when.
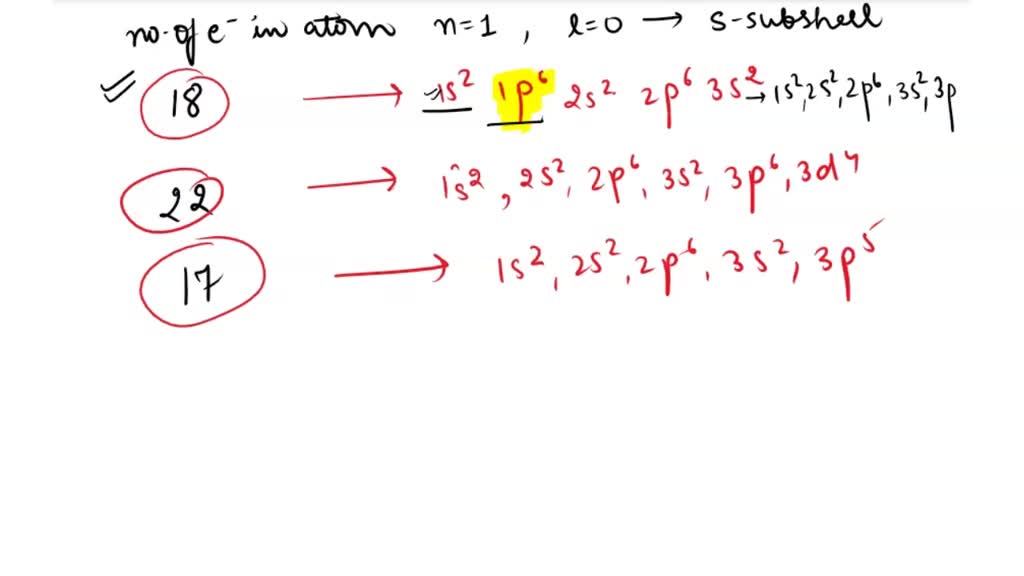
Another common pitfall is the Pauli exclusion principle. This one’s like a strict apartment rule: each “room” (orbital) can only hold a maximum of two electrons, and they have to have opposite “spins” – think of them as spinning in opposite directions, like two dancers doing a perfectly synchronized pirouette, but in opposite ways. If you try to cram three electrons into one orbital, or put two electrons in with the same spin, that’s a no-go. It’s like trying to fit three people into a single-person kayak – chaos!
Then there’s Hund’s rule. This one’s a bit more about fairness and spreading out. If you have multiple orbitals with the same energy (like several identical rooms on a floor), electrons will first occupy each of these orbitals singly, with parallel spins, before pairing up. It’s like when you’re at a buffet, and everyone takes a little bit of everything before going back for seconds. Electrons tend to spread out and take their own “spot” first before they have to double up.
Forgetting these simple-sounding rules can lead to an incorrect electron configuration. It's like following a recipe but forgetting to add the baking soda – your cake might not rise properly!

Why Should We Even Care? The Real-World Ripple Effect
Okay, so we’ve touched on how electrons are arranged. Why is this so important that we’d even bother talking about mistakes? Because it’s the foundation for so much of what makes our world work. Think about it: every single material you interact with, from your smartphone to the water you drink, is made of atoms. And how those atoms behave, how they connect and form molecules, is all dictated by their electron configurations.
When scientists get electron configurations right, they can predict how elements will react. This is HUGE. It’s how we develop new medicines, create stronger and lighter materials for airplanes and cars, design efficient solar panels, and even understand the intricate processes happening inside our own bodies. It’s like knowing the right ingredients and the right order to mix them in to bake the perfect loaf of bread versus a dense, unappetizing brick.
Let’s say you’re trying to create a new type of battery. The performance of that battery depends entirely on how the electrons in the materials behave and move. If you get the electron configuration wrong, you might end up with a battery that either doesn't hold a charge, charges too slowly, or is even unsafe. That’s a pretty big oopsie, especially when you’re relying on your phone to get you through the day!

Or consider the colors we see! The vibrant reds of a sunset or the calming blues of the ocean are all thanks to how electrons in atoms absorb and emit light. Different electron arrangements lead to different energy transitions, and those transitions correspond to different wavelengths of light. Get the electron arrangement wrong, and you might not get that dazzling spectrum you’re expecting.
Even something as fundamental as chemical bonding – why a hydrogen atom happily teams up with an oxygen atom to form water (H₂O) – is all about electrons wanting to achieve a stable configuration, like a group of friends wanting to sit together on a comfy couch. If you misunderstand how those electrons want to arrange themselves, you won’t understand why water is H₂O and not, say, H₃O or HO₂.
Spotting the Glitches: A Little Detective Work
So, how do we identify these electron configuration errors? It’s like being a detective looking for clues. We often use something called spectroscopy, which is a fancy word for studying how light interacts with matter. When electrons jump between energy levels, they either absorb or emit specific amounts of light. By looking at the pattern of light absorbed or emitted (think of it as an element's unique fingerprint), scientists can deduce the electron configuration. If the observed fingerprint doesn't match the predicted configuration, you’ve found an error!
It’s kind of like trying to guess someone’s favorite song by the humming they do. If they’re humming a tune you don’t recognize, you might be missing a piece of the puzzle. Spectroscopy helps us "hear" the electron's favorite tunes.
Another way is through chemical reactivity. If an element is behaving in a way that doesn’t make sense based on its predicted electron configuration – maybe it’s bonding with elements it shouldn’t or not reacting when it’s expected to – that’s a big red flag. It’s like expecting your dog to fetch a stick and instead it starts trying to knit a sweater. Something’s up!
Understanding these little quantum mechanics hiccups isn’t just for super-scientists in labs. It’s about appreciating the incredible complexity and beauty of the universe at its smallest scales. Every time we marvel at a piece of technology or enjoy the natural world, we’re indirectly benefiting from the meticulous work of understanding these tiny electron arrangements. So, the next time you’re marveling at the vibrant colors of a flower or the efficiency of your phone, give a little nod to those electrons and their sometimes-tricky configurations. They’re the unsung heroes of our everyday lives!
