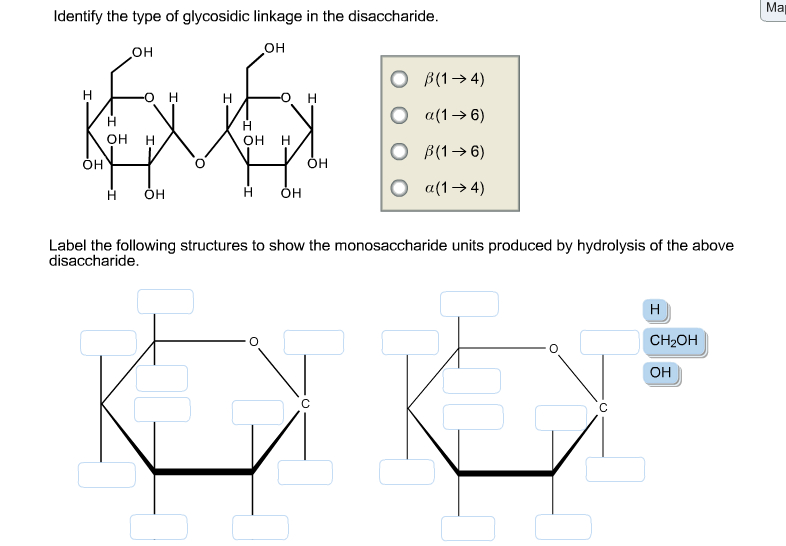
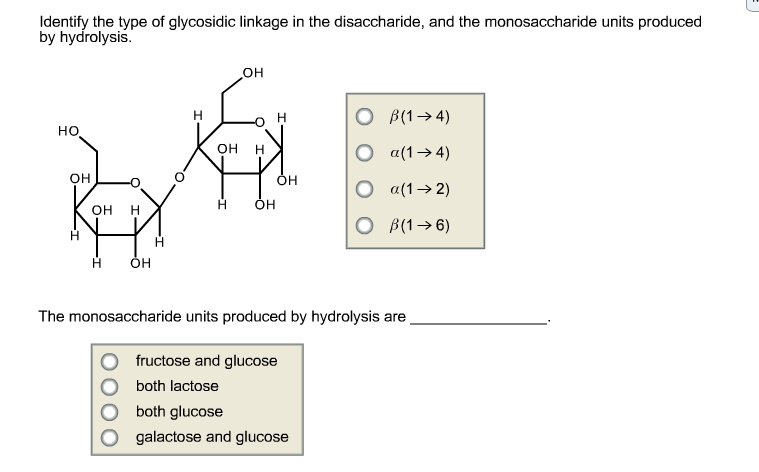
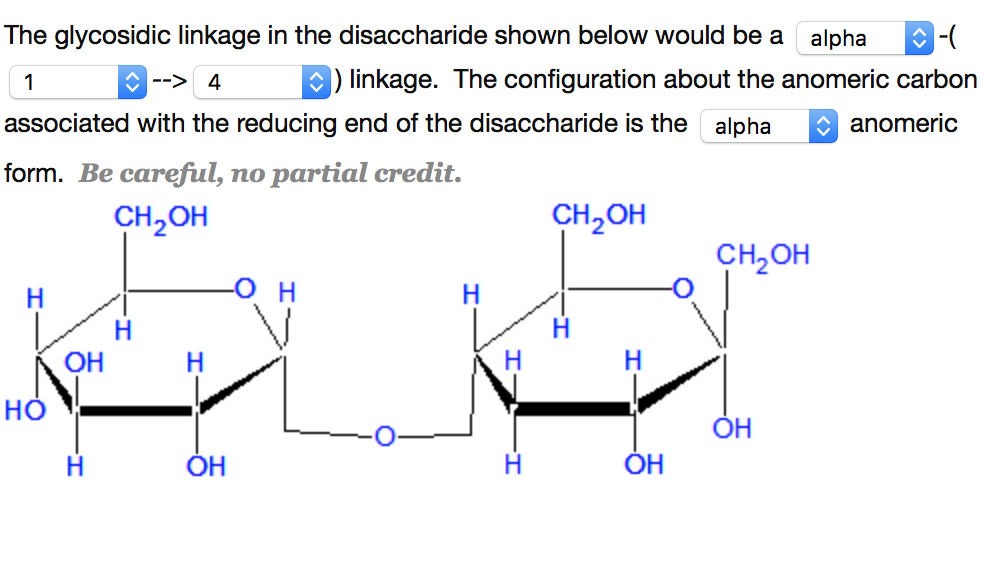
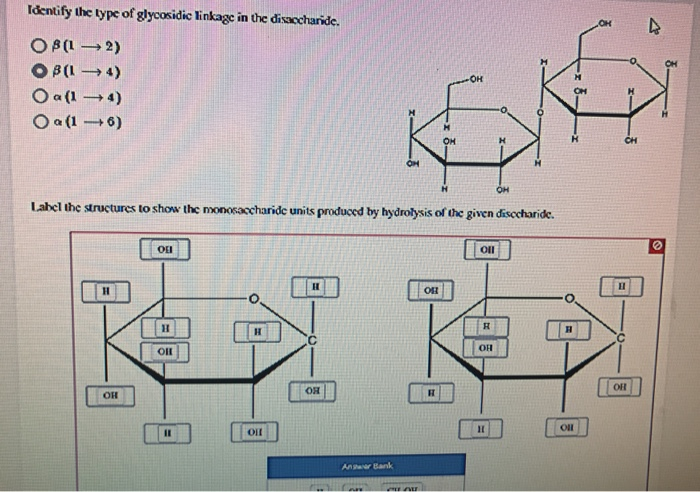
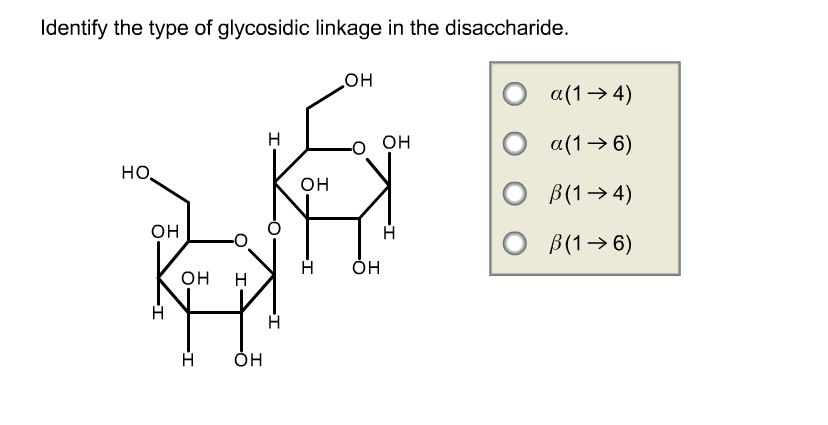
Identify The Type Of Glycosidic Linkage In The Disaccharide
Hey there, sugar fiends and science nerds! Ever wonder what makes your favorite sweet treats… well, sweet? It’s not just magic, though it might feel like it sometimes. It’s all about tiny chemical connections, and today, we’re diving headfirst into the fascinating world of glycosidic linkages. Sounds fancy, right? But trust me, it’s way cooler than it sounds. We’re gonna break down how these sugar buddies hook up in disaccharides. Think of it as speed dating for sugars!
So, what’s a disaccharide? Easy peasy. It’s just two simple sugars, like two little LEGO bricks, clicked together. Think of that sweet, sweet table sugar you use every day – that’s sucrose! It’s made of glucose and fructose holding hands. But how do they hold hands? That’s where our star, the glycosidic linkage, comes in.
Imagine two sugar molecules, both with a hydroxyl group (-OH) just hanging out, looking for a connection. They’re like two single molecules at a party, and they decide to pair up. The glycosidic linkage is the specific way they join hands, forming a bond and kicking out a water molecule in the process. Poof! Two become one (sort of).
The Alpha and the Beta Tango
Now, here’s where it gets a little quirky. Sugars aren’t just simple circles. They have little structural differences. The most important distinction for our glycosidic linkage party is whether the connection is alpha (α) or beta (β). Think of it like a handshake. An alpha handshake is one way, and a beta handshake is another. They look almost the same, but the little tilt of the connection makes a HUGE difference.
This alpha-beta thing happens at a specific carbon atom on the sugar ring, usually carbon number 1. This is called the anomeric carbon. It’s like the prime real estate for sugar bonding. The hydroxyl group on the anomeric carbon is the one that gets involved in the linkage.
So, if the hydroxyl group on the anomeric carbon of one sugar links with a hydroxyl group on another sugar in the alpha configuration, we call it an alpha glycosidic linkage. If it’s in the beta configuration, you guessed it – it’s a beta glycosidic linkage.
Why does this matter? Oh, you have no idea! It’s the difference between a delicious treat and something your body can’t even digest. It’s like trying to fit a square peg in a round hole, but for your digestive enzymes.
Let’s Meet the Disaccharide Stars!
Let’s look at some famous disaccharides and their unique handshake styles. This is where the fun really begins!
Maltose: The Beer Maker’s Buddy
First up, we have maltose. Ever enjoyed a nice cold beer? Or maybe some malted milk balls? Thank maltose! It’s two glucose molecules having a little rendezvous. But what kind of handshake do they do? They form an α-1,4 glycosidic linkage.
What does that mean? Let’s break it down. The α tells us it’s an alpha configuration at the anomeric carbon of the first glucose. The 1,4 tells us where on the second glucose molecule the linkage happens. It’s connecting the anomeric carbon (carbon 1) of the first glucose to carbon 4 of the second glucose. It's like they’re holding hands across the table, with one hand reaching out from carbon 1 and the other hand grabbing carbon 4.
This α-1,4 linkage is super important because it’s found in starch, our energy storage molecule in plants. Our bodies have enzymes that are really good at breaking these down. That’s why we can get energy from bread and potatoes!
Lactose: The Milk Mystery
Next, let’s talk about lactose. This is the sugar found in milk. If you’re lactose intolerant, you know all about the drama this disaccharide can cause! Lactose is made of one glucose molecule and one galactose molecule. Galactose is like glucose’s twin sister, with a slight difference in structure.
Now, for the linkage: lactose has a β-1,4 glycosidic linkage. This means the anomeric carbon of galactose (in the beta configuration, remember?) links to carbon 4 of glucose. See the difference? The beta handshake! This subtle change is a big deal for how our bodies process it.
Most people have an enzyme called lactase that can break this β-1,4 linkage. But if you don’t have enough lactase, the lactose just hangs out in your gut, causing all sorts of... musical interludes. It's a classic case of the wrong linkage for the available enzymes!
Sucrose: The Sweet Deceiver
And finally, the queen of sweetness, sucrose! Yes, that’s your table sugar, the stuff that makes cookies, cakes, and, well, everything delicious! Sucrose is a team-up of glucose and fructose. Fructose is a bit of a different beast, a five-membered ring instead of a six-membered one. This makes things a little more interesting.
Sucrose has a really special linkage: an α,β-1,2 glycosidic linkage. Whoa, what does that mean? It means the anomeric carbon of glucose (in the alpha configuration) is linked to the anomeric carbon of fructose (in the beta configuration). Yep, they’re both using their prime real estate to connect! And it’s a 1,2 linkage because it’s carbon 1 of glucose and carbon 2 of fructose.
This linkage is super stable. It’s like they’ve formed an unbreakable bond. This is why sucrose is so sweet and why it doesn’t readily break down. It takes specific enzymes, like sucrase, in our digestive system to get the job done. If you’ve ever tried to digest something super sugary and felt a little sluggish, sucrose might be part of the reason!
Why Is This Even Fun?!
Okay, so why should you care about alpha and beta linkages? Because it's all about structure dictates function! These tiny differences in how sugar molecules connect are the foundation of life. They determine how we store energy (starch vs. glycogen), what we can eat (lactose intolerance, anyone?), and even the structure of plants (cellulose!).
It's like a secret code written in sugar. The enzymes in our bodies are the decoders. If the code is right (like the α-1,4 in starch), they can read it and give us energy. If the code is wrong for them (like the β-1,4 in cellulose, which is what plant cell walls are made of), they can’t break it down. That's why we can eat a potato but can't digest wood. Nature's got a sense of humor!
Think about it: a single hydroxyl group tilted just a smidge differently can mean the difference between a source of instant energy and something your body can’t use at all. How wild is that? It’s the ultimate proof that the little things matter. So, the next time you’re enjoying something sweet, give a little nod to the amazing world of glycosidic linkages. They’re the unsung heroes of deliciousness and the backbone of so many biological processes. Pretty cool, right?
