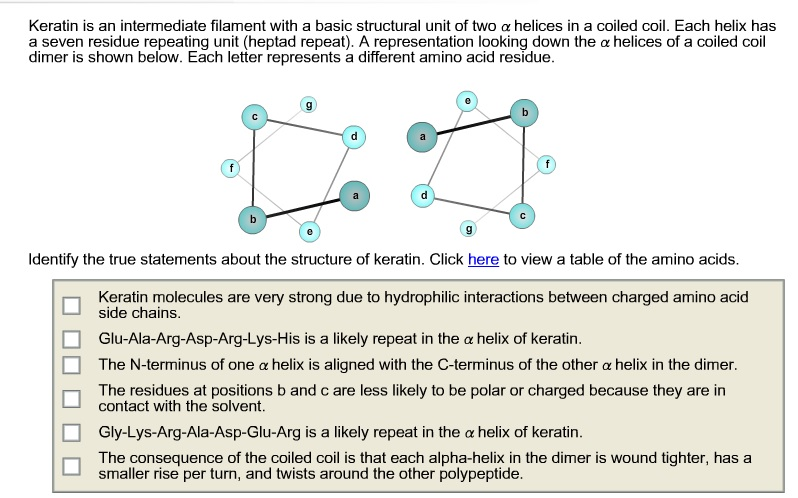
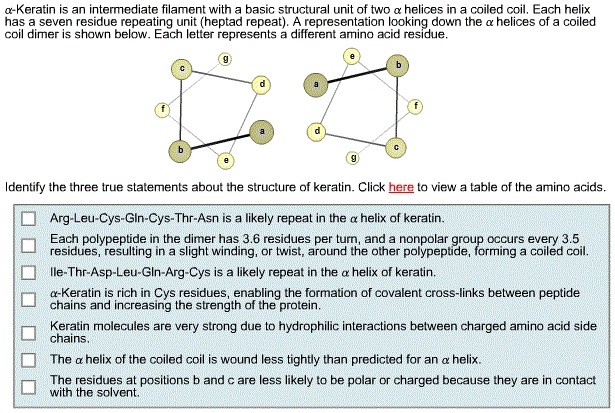
Identify The Three True Statements About The Structure Of Keratin

Alright, so you're sitting there, maybe with a latte that’s costing you more than your rent (don't judge!), and you’re wondering, "What's the deal with my hair? My nails? My glorious mane? Is it magic? Pixie dust? Nope, my friends, it’s keratin! And let me tell you, this protein is the unsung hero of your everyday fabulousness.
We’re diving deep, folks, into the nitty-gritty of this amazing molecule. Think of it like a construction site, but instead of tiny bricklayers, we’ve got some seriously impressive protein chains doing all the heavy lifting. And just like any good construction project, there are blueprints, there are materials, and there are some surprisingly… structured elements at play. So, grab another croissant, because we're about to reveal three true statements about the structure of keratin, sprinkled with a generous dose of disbelief and maybe a few snorts of laughter.
The Backbone of Your Beauty
First things first, let's acknowledge the sheer resilience of keratin. Seriously, this stuff is tough. It’s the reason why your hair can withstand a vigorous brushing (most of the time), why your nails don’t just snap off when you try to open a stubborn jar (again, most of the time). It's basically nature's Super Glue, but way more stylish.
Now, understanding keratin's structure is like trying to understand why your cat suddenly decides to sprint across the room at 3 AM. It seems chaotic, but there's a method to the madness. We’re going to break down the architecture, piece by piece, and you’ll walk away feeling like a keratin connoisseur. No more nodding along blankly when someone mentions alpha-helices, oh no. You'll be dropping knowledge bombs like it's your job.
Statement 1: It’s All About the Alpha-Helices (Like Tiny, Coiled Springs!)
So, the very first, incredibly important truth bomb about keratin's structure is that it's primarily built from these things called alpha-helices. Now, don't let the fancy name scare you. Imagine a slinky, a really, really tiny, protein slinky. That's kind of what an alpha-helix is. It's a specific way a chain of amino acids – the building blocks of proteins – coils up on itself. Think of it as the most efficient way for that protein chain to pack itself neatly.
These alpha-helices aren't just lounging around individually, though. Oh no. They're like the initial construction crew, getting their shapes. They then twist and turn and interlock with other alpha-helices. It's a beautiful, coiled dance. This coiling gives keratin a lot of its inherent strength and flexibility. It's like having a rope made of tiny, coiled springs – it can stretch a bit, but it’s got a lot of underlying power.

And get this: the way these helices are arranged is super important. They don't just randomly coil; they coil in specific directions, creating a sort of “supercoil” or a protofibril. It's like taking those tiny slinkies and twisting them together into a thicker, stronger rope. If you’ve ever seen those close-ups of hair or nails under a microscope (and who hasn’t, right? Just me?), you’re seeing the results of this incredible helical arrangement.
So, the first truth: Keratin’s structure is largely based on coiled protein chains called alpha-helices. It’s the foundational coil that makes everything else possible. Without these little slinkies, your hair would be… well, less than fabulous. Probably more like a pile of limp spaghetti. And nobody wants that.
Statement 2: Disulfide Bonds are the Super Glue Holding It All Together
Okay, so we have our coiled alpha-helices. But what keeps them from just unraveling like a cheap sweater? Enter the unsung heroes, the real MVP's of keratin’s structural integrity: disulfide bonds. These are chemical bonds that form between sulfur atoms in the amino acid cysteine, which is found in keratin.
Think of them as the tiny, incredibly strong rivets or welds that hold our protein coils together. They’re like the super glue that nature uses to make sure your hair doesn't just fall apart at the slightest provocation. These bonds are strong, people. They’re the reason perms and straightening treatments work – they break and then reform these disulfide bonds to change the shape of your hair. It’s basically molecular manipulation for beauty!
This is where the real "toughness" of keratin comes in. The more disulfide bonds there are, the stronger and more rigid the keratin structure will be. That's why your fingernails are much harder than your hair. They've got more of these little sulfur-powered super-glued connections. Imagine building a bridge: you wouldn't just use twine, right? You'd use steel beams and rivets. Disulfide bonds are the keratin equivalent of those steel rivets, making sure everything stays put.
So, the second truth: Disulfide bonds are crucial for linking and stabilizing the coiled protein structures in keratin. They are the molecular equivalent of industrial-strength super glue, making keratin resilient and durable. Without them, your hair would be flimsier than a politician’s promise.

Statement 3: It’s a Fibrous Protein, Not a Globular One (Think Twine vs. Ball)
Now, for our final, mind-blowing truth: Keratin isn't a 'globular' protein. You know, like the proteins in your blood that are all round and compact, like tiny little protein marbles? Nope. Keratin is a fibrous protein. This means its structure is elongated and thread-like, rather than spherical.
This fibrous nature is directly related to how those alpha-helices and disulfide bonds assemble. They don’t just clump together into a ball. Instead, they line up, parallel to each other, forming long, strong fibers. Think of it like making yarn. You take those individual strands (the alpha-helices) and twist them together (with the help of disulfide bonds) to create a continuous, strong thread. Then, you take those threads and weave them together to make fabric. Keratin does something very similar.
This fibrous structure is what gives keratin its tensile strength – its ability to resist being pulled apart. It's this long, organized, thread-like arrangement that makes your hair strong enough to, you know, be hair. It’s designed for structural support, not for carrying out rapid chemical reactions like some of those globular proteins do. Keratin is the quiet, strong workhorse of the protein world.
So, the third truth: Keratin is a fibrous protein, characterized by its long, elongated structure, which contributes to its strength and resilience. It's built for structure, not for a fancy ballet performance. It’s the humble, yet mighty, thread that weaves through your skin, hair, and nails, keeping you looking (and feeling) put together.
The Takeaway: You’re Basically Made of Tiny, Coiled, Super-Glued Threads!
So there you have it! Three fundamental truths about the structure of keratin that explain why you can confidently flip your hair, style it, or even, dare I say, accidentally snag a nail. It’s all thanks to these amazing alpha-helices, the incredibly strong disulfide bonds, and the overall fibrous nature of this protein.
Next time you catch your reflection and admire your shiny hair or your perfectly manicured nails, give a little nod to keratin. It's working overtime, silently building the scaffolding of your beauty. And who knows, maybe you’ll even whisper a thank you to those tiny, coiled, super-glued threads. They've earned it. Now, about that second latte…
