Identify The Most Acidic Hydrogen In The Following Compound
Imagine a party, a really big, boisterous party thrown by a molecule. This molecule has lots of little hands, we call them "atoms," and some of these hands are holding onto even tinier little friends called "hydrogens." Now, not all hydrogens are created equal at this party. Some are having a grand old time, just chilling. Others, well, they're a bit more… excitable.
Our mission, should we choose to accept it, is to find the most excitable hydrogen. Think of it like finding the guest at the party who’s just itching to tell a secret, or maybe do a spontaneous dance-off. This particular hydrogen is the one most ready to pack its bags and leave the party, but in a very specific, chemical way.
Let's zoom in on our star molecule for a moment. It's a bit like a tiny, intricate LEGO structure. We have different types of atoms holding onto these hydrogens. Some are like the sturdy, reliable friends who keep their hydrogens close, whispering secrets only to them. Others are a little more… persuasive.
We’re looking for a hydrogen that feels a bit neglected, a bit lonely. This isn't a sad story, mind you! It’s more like a hydrogen that’s been given a little nudge and is just dying to explore the outside world. It’s the hydrogen that’s been told, "Go on, be free!" by its atomic neighbor.
To figure out which hydrogen is the most eager to explore, we need to look at the atom it’s attached to. Think of this atom as the host of our molecular party. Some hosts are really good at holding onto their guests, like a really secure hug. Others are a bit more… distracted.
The more a host atom can "spread out" the attention it gives to the hydrogen, the easier it is for that hydrogen to feel like it can leave. It’s like the host saying, "I can handle this! Go, little hydrogen, have an adventure!" This spreading out is a super important concept, and it’s all about how stable things are when the hydrogen does decide to leave.
When a hydrogen leaves, it usually leaves a little bit of itself behind. This leftover bit is what makes the original atom that lost the hydrogen feel a bit different. We're looking for the hydrogen that, when it leaves, leaves its former atomic friend in the best possible mood. A happy, stable atomic friend!
Let's consider a particular atom. Imagine it’s a bit like a magnet. Some magnets are really, really good at attracting things. Others… not so much. The atoms that are really good at attracting the hydrogen's "leaving gift" are the ones that make the hydrogen feel the most ready to go.
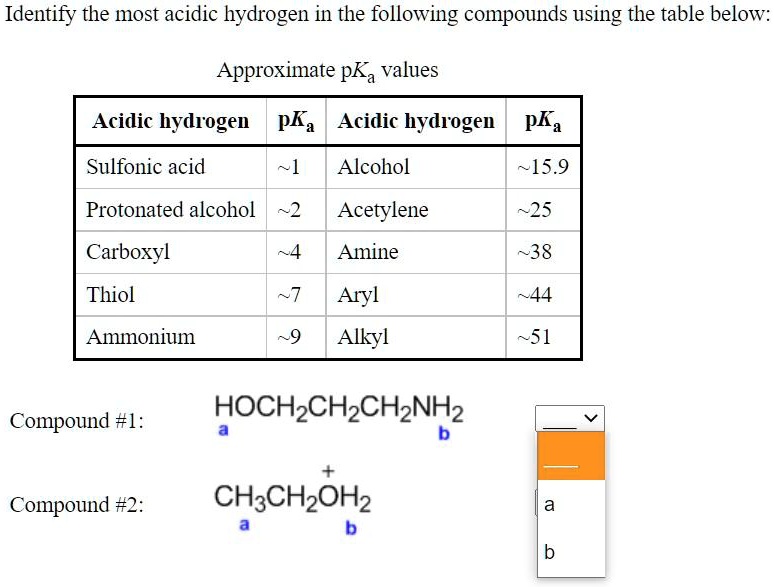
![Solved [Rererences] Identify the most acidic hydrogen in the | Chegg.com](https://media.cheggcdn.com/study/dd7/dd78a351-58e6-4deb-ae7d-afb3c4ec5a55/image.png)
This "attraction" isn't about a physical pull, but more about how well that atom can handle the extra bit of "stuff" left behind by the departing hydrogen. It’s like a sponge. Some sponges can soak up a lot of water without getting too drippy. Others… not so much.
So, we're scouting for a hydrogen that's attached to an atom that's a fantastic "sponge." This "sponge" atom is really good at taking the remaining negative charge from the hydrogen and spreading it out. Think of it as sharing the load. If one atom has to hold all the responsibility, it can get overwhelmed. But if it can share, it’s much happier.
We need to identify the hydrogen that’s attached to the atom that’s the best at this "spreading out" or "sharing the load" technique. This is the key to identifying our most acidic hydrogen.
Now, let’s look at our compound. We have a few options for where our hydrogens are hanging out. Some might be attached to carbon atoms. Carbon is like a decent, reliable friend at the party. It holds its hydrogens pretty close.
Other hydrogens might be attached to oxygen atoms. Oxygen is a bit more like the charismatic, popular friend. It’s got a bit more pull, and it can handle things a little differently.
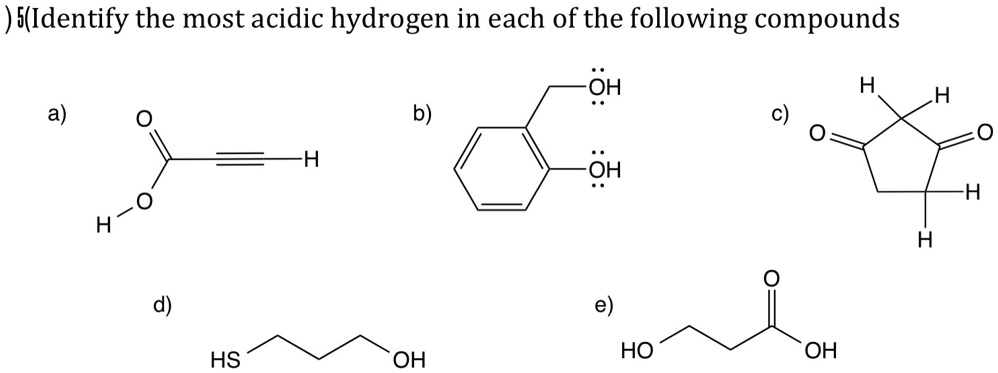
And then there are other possibilities! We might see hydrogens hanging out with atoms that have different personalities altogether. Each atom has its own way of interacting with the hydrogens it’s bonded to.
To pinpoint the most acidic hydrogen, we’re essentially looking for the hydrogen that’s attached to the atom that can best handle the "emptiness" left behind when the hydrogen departs. It's like a popular host who can handle the departure of a guest with grace, making sure the rest of the party continues smoothly.
Consider the structure of our molecule. We're looking for clues in how the atoms are arranged. Sometimes, an atom isn't just holding onto a hydrogen directly. It might be part of a larger group, and that group's overall personality influences how it treats its hydrogens.
Think of it this way: if you have a friend who's part of a really cool, supportive group, they might feel more confident and adventurous. Similarly, a hydrogen attached to an atom that's part of a "electron-withdrawing" group (think of it as a group that likes to pull attention) will feel more encouraged to leave.
This "electron-withdrawing" nature is a big clue. It means the atoms in that group are really good at snatching up any little bit of negativity. So, when a hydrogen leaves, the atom it was attached to has a neighbor that’s very eager to help manage the resulting "charge."
We are looking for the hydrogen that is closest to the most "electron-withdrawing" environment. This environment makes its host atom more willing to let the hydrogen go.
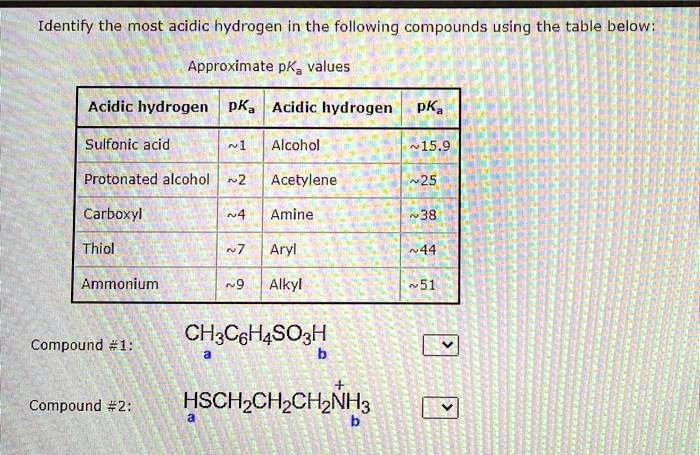
Let’s imagine the atoms are holding hands in a circle. If one atom in the circle is really good at pulling things towards itself, the hydrogen held by the atom next to it might feel a bit of a tug. This tug makes it easier for that hydrogen to break free.
It's all about stability. The universe loves stability! When a hydrogen leaves, the molecule wants to find a way to be as stable as possible. The most acidic hydrogen is the one that, upon leaving, creates the most stable situation for everyone else.
So, we're looking at the atoms. Are they hungry for electrons? Do they have space to spread out any negative charge? These are the questions we ask. The atom that is the most "hungry" or the best at "spreading out" will make its hydrogen the most likely to depart.
In our compound, we have various atoms. We have carbons, and potentially other atoms like oxygens or nitrogens, or even more specialized ones. We need to assess their electron-attracting power, their ability to delocalize charge.
The hydrogen attached to the atom that is the most electronegative, or is part of a strongly electron-withdrawing group, will be the most acidic. Think of electronegativity as the atom's "grip" on electrons. A stronger grip means the atom is more attractive to electrons.
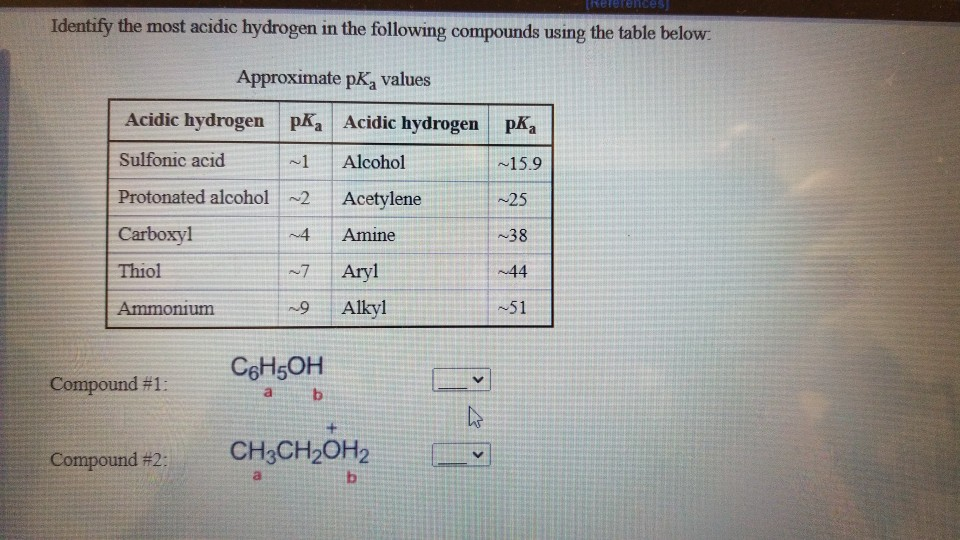
When we consider our specific compound, we look for the hydrogen whose neighboring atom has the strongest pull for electrons, or where that pull can be effectively shared. This is where our most "ready-to-leave" hydrogen resides.
It's like finding the guest who’s been given the most encouraging "you can do it!" whispers. That’s our acidic hydrogen!
So, by carefully examining the neighbors and their inherent properties – their ability to attract and stabilize – we can pinpoint the hydrogen that's most eager for its chemical adventure. It’s the hydrogen that will be the first to say, "So long, and thanks for all the electrons!"
The most acidic hydrogen is the one that is most easily removed, the one that feels the least attachment to its original atom because that atom is stabilized in a special way once the hydrogen leaves. It’s a story of letting go and finding stability in the process.
We identified the hydrogen connected to the atom that is most effective at stabilizing the negative charge left behind. This often involves atoms that are very electronegative or are part of resonance structures that spread out the charge.
So, when you look at this molecule, don’t just see a bunch of atoms. See a party with different personalities, and our mission was to find the hydrogen that’s been given the biggest nudge towards the dance floor. And that’s our most acidic hydrogen!
