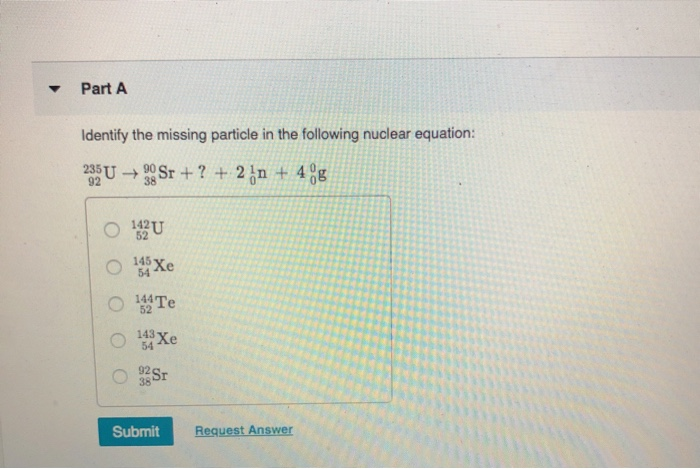
Identify The Missing Particle In The Following Nuclear Equation

Okay, science friends! Let’s talk nuclear reactions. Sounds super serious, right? But honestly, it’s like a cosmic puzzle. And today, we’ve got a missing piece.
Imagine a tiny, tiny explosion. Or maybe a very, very small magic trick. That’s kind of what happens when atoms decide to… well, rearrange themselves. We’re talking about nuclear equations. They’re like recipes for atomic transformations.
And every good recipe has all its ingredients. What if one is missing? That’s our little mystery today!
The Nuclear Equation Mystery
So, here’s the equation. Keep an eye on it. We’ve got stuff on one side, and stuff on the other. Think of it like balancing your checkbook, but with protons and neutrons.
On the left side, we have:
U-235 + n → ?? + Ba-141 + 3n
See that question mark? That’s our star player. The missing particle. Our whodunnit of the atom world.
Let’s break it down, super chill. We’ve got Uranium-235 (that’s the U-235). It’s a pretty big atom. Think of it as a bit of a drama queen in the periodic table. It’s radioactive, meaning it’s always a little antsy, ready for a change.
Then we have a neutron (n). Just a lone little neutron. It’s like the catalyst, the spark that gets the whole reaction going. It bumps into the Uranium-235.
What happens next? BOOM! Well, a nuclear BOOM. The Uranium atom gets excited. It splits apart. It’s called nuclear fission. It’s like a piñata breaking open, but instead of candy, you get smaller atoms and more neutrons.
On the right side, we see the results. We’ve got Barium-141 (Ba-141). That’s one of the new, smaller atoms. And then we have three more neutrons (3n). These are the bonus neutrons, the ones that can go off and start more fission reactions. This is how a chain reaction gets started! Pretty wild, huh?
The Balancing Act
Here’s where the fun really begins. In nuclear equations, two things must be conserved. They’re like the unbreakable rules of atomic physics. Number one: the total number of particles. Number two: the total electric charge.
Think of it as keeping score. The total number of protons and neutrons on the left side of the arrow has to equal the total number on the right side. Same goes for the total charge. Easy peasy, right?
Let’s look at our equation again:
U-235 + n → ?? + Ba-141 + 3n
First, the number of particles. This is the mass number. It’s the big number in the atom’s name (like 235 for Uranium). It represents the protons and neutrons combined.
On the left: Uranium-235 has a mass number of 235. The neutron has a mass number of 1. So, 235 + 1 = 236. We have 236 particles on the left.

On the right: Barium-141 has a mass number of 141. The three neutrons each have a mass number of 1. So, 141 + (3 * 1) = 141 + 3 = 144. We have 144 particles on the right.
Uh oh. 236 on the left, 144 on the right. That doesn’t balance! Our missing particle has to account for the difference.
The difference is 236 - 144 = 92. So, our missing particle needs to have a mass number of 92.
Now, About That Charge…
This is where it gets a little more interesting. Atoms have protons, which are positively charged. Neutrons are neutral, no charge. So, the charge comes from the protons. We use the atomic number for this. It’s the number of protons in an atom’s nucleus.
Let’s check the atomic numbers (the little number usually written as a subscript before the element symbol). If you don’t have it memorized, that’s totally fine! We can always look it up. It’s like having a cheat sheet for the universe.
Uranium (U) has an atomic number of 92. This means it has 92 protons.
Neutrons (n) have no charge, so their atomic number is 0.

On the left side: Uranium (92) + neutron (0) = 92. We have a total charge of 92.
On the right side: Barium (Ba) has an atomic number of 56. So, Barium-141 contributes a charge of 56. The three neutrons contribute 0 charge.
So, on the right, we have 56 + 0 = 56. We have a total charge of 56.
Again, not balanced! 92 on the left, 56 on the right. The difference is 92 - 56 = 36. Our missing particle needs to have a charge of 36.
Putting The Pieces Together
So, our missing particle needs:
- A mass number of 92
- A charge (atomic number) of 36
Now, we just need to find the element on the periodic table that has an atomic number of 36. Drumroll, please… it’s Krypton (Kr)!
And Krypton, with an atomic number of 36, has a common isotope with a mass number of 92. So, our missing particle is Krypton-92 (Kr-92).

Let’s plug it back in:
U-235 + n → Kr-92 + Ba-141 + 3n
Let’s double-check:
- Mass Numbers: 235 (U) + 1 (n) = 236. On the other side: 92 (Kr) + 141 (Ba) + 3 (n) = 92 + 141 + 3 = 236. Balanced!
- Atomic Numbers (Charge): 92 (U) + 0 (n) = 92. On the other side: 36 (Kr) + 56 (Ba) + 0 (n) = 36 + 56 + 0 = 92. Balanced!
Ta-da! We solved it. The missing particle is Krypton-92.
Why Is This Fun?
Because it’s like cracking a secret code! Atoms are constantly doing their thing, transforming, releasing energy. And understanding these equations helps us understand everything from how power plants work to how stars shine. It’s the language of the universe, just in tiny, tiny letters.
Plus, thinking about U-235 as a drama queen? Or neutrons as tiny sparks? It makes the abstract tangible, and dare I say, a little bit cute.
These reactions are responsible for some of the most powerful forces we know. But the math behind it is just… elegant. It’s about conservation, about everything adding up perfectly in the end. It’s a little bit of order in the chaos of atomic particles.
So next time you hear about nuclear reactions, don’t just think of big bangs. Think of puzzles. Think of missing pieces. And remember that even in the most powerful processes, there’s a beautiful, balanced equation waiting to be discovered. It’s fun to know these things, right? It makes you feel a little bit like a cosmic detective.
