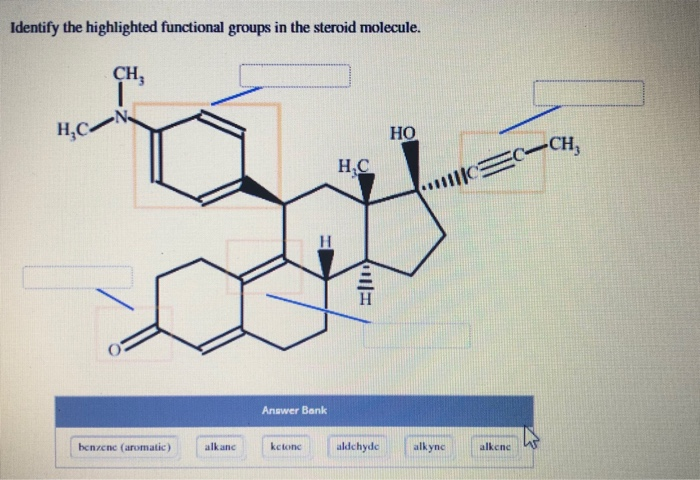
Identify The Highlighted Functional Groups In The Steroid Molecule

Ever wondered what makes certain molecules so darn interesting? Well, get ready to peek into the wacky world of steroids! These aren't the ones you hear about in the gym gossip. We're talking about the super cool building blocks of life. It’s like a secret code that nature uses to build some truly amazing things.
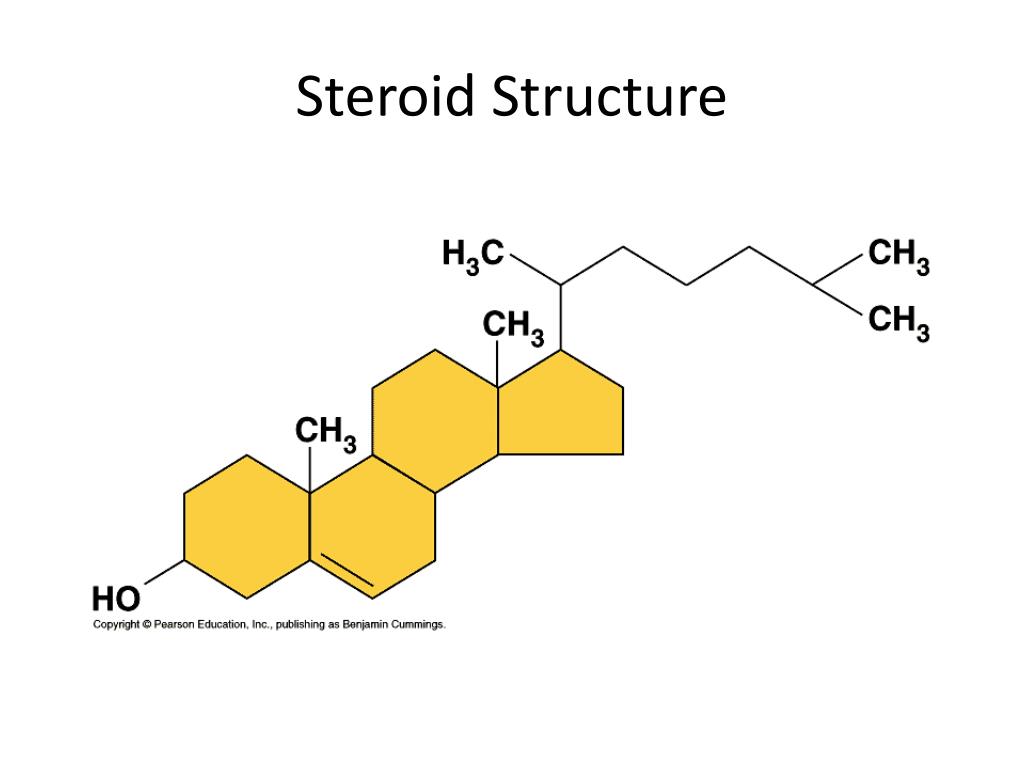
Imagine a molecule with a special ring structure. This is the basic blueprint for all steroids. It's like a classic recipe that gets tweaked in all sorts of fun ways. This core shape is what gives steroids their unique identity and power. It's the foundation for their incredible diversity.
Now, what makes a steroid special? It's all about the little add-ons, the tiny bits and bobs that get attached to that basic ring. These are called functional groups. They're like the decorations on a cake, or the different flavors you can add to a basic vanilla ice cream. These groups are the real stars of the show!
Let's zoom in on a typical steroid molecule and see what’s decorating its rings. Think of it like a treasure hunt! We’re looking for those special chemical features that give each steroid its own personality. Each one has a job to do, and it's fascinating to see how they work together.
One of the most common and important players is the hydroxyl group. You can spot it by its signature -OH. It's like a little hook that loves to interact with water and other molecules. This group is everywhere in biology, and in steroids, it can make a big difference in how the molecule behaves.
Think of that -OH group like a tiny handshake. It can grab onto other things, or let go. This makes it super useful for the body. It's involved in all sorts of chemical reactions. It's a real team player, always ready to lend a hand.
Next up, we have the keto group. This one looks like a double bond to oxygen, a C=O. It’s a bit more stern-looking than the hydroxyl group, but just as important. This group is also a hub for chemical activity. It can be a target for other molecules to bump into.
The keto group is like a little magnet for certain types of reactions. It’s a bit more reactive than some other parts of the molecule. This makes it a key player in how steroids are made and broken down in the body. It’s a crucial part of their story.
Sometimes, you’ll see a steroid sporting a carbonyl group. This is very similar to the keto group, but can also appear as part of a larger structure like an ester or amide. For our purposes today, let’s focus on the direct keto form. It’s the simplest and most common way we see this important feature.

These carbonyls are like power outlets. They can provide the energy or the reactivity needed for certain biological processes. They are essential for many of the functions steroids perform. Without them, things just wouldn't get done!
Then there’s the methyl group. This is a simple little guy, just a carbon atom with three hydrogens, -CH3. It might seem small and insignificant, but don't underestimate it! Even these tiny additions can change the shape and behavior of a steroid.
Think of methyl groups as little bumps or extensions. They can affect how well a steroid fits into its target. It’s like trying to fit a puzzle piece with a slightly different edge. This subtle difference can change everything.
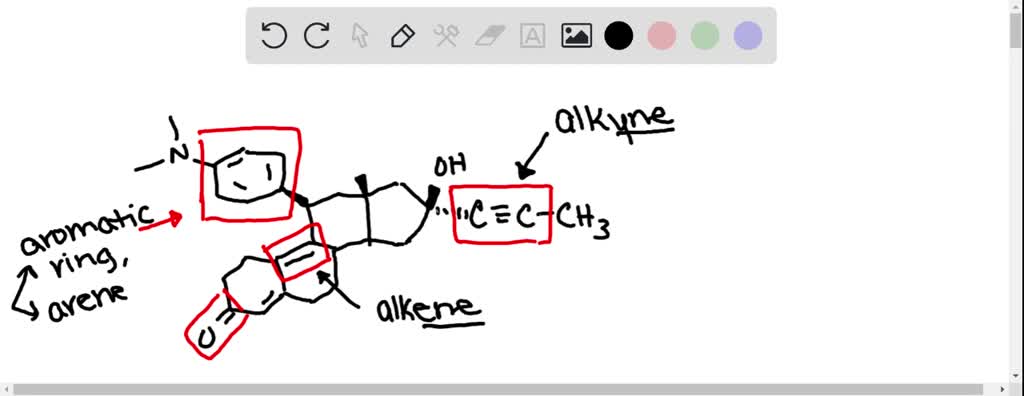
We might also find alkene groups. These are double bonds between carbon atoms, like C=C. These are points of extra "unsaturation" in the molecule. They are often found in the rings themselves, or in side chains attached to the rings.
These double bonds are like little hinges. They allow for some flexibility in the molecule's structure. They can also be sites for chemical reactions. It's like having a flexible joint that can bend and twist.
Now, let's imagine we’re looking at a specific steroid. Let's say we're admiring a molecule like cholesterol. Cholesterol is a famous steroid, and it has a few key functional groups that make it so important. See that -OH group attached to one of the rings? That’s our hydroxyl friend!
That hydroxyl group on cholesterol is super important. It makes cholesterol slightly soluble in water. This is crucial because our bodies are mostly water! It helps cholesterol travel around in our blood. It's like a tiny raft that lets it float along.
And what about the rest of the cholesterol molecule? It's mostly made up of carbon and hydrogen atoms, forming those familiar rings and a wiggly tail. This long hydrocarbon tail is not polar. It likes to stick to other non-polar things. It’s the part that loves to hide from water.
So, cholesterol is a bit of a split personality! It has a water-loving head (the -OH) and a water-hating tail. This dual nature is what makes it so useful in cell membranes. It's like a tiny bouncer, controlling what gets in and out of cells.
Let's switch gears and look at a different steroid, perhaps something like a sex hormone. These hormones, like testosterone or estrogen, are steroids too! They have that same basic ring structure, but their functional groups are arranged just a little bit differently.
Testosterone, for instance, has a keto group (C=O) and a hydroxyl group (-OH). These groups are key to its function as a male sex hormone. They allow it to bind to specific receptors in the body. It’s like having a special key that only fits a particular lock.
Estrogen, on the other hand, has a slightly different setup. It often has a hydroxyl group and an aromatic ring. That aromatic ring is a special kind of ring structure, very stable and flat. It’s like a rigid platform that helps the molecule sit just right.
These subtle differences in functional groups are what make each steroid unique. They dictate how the molecule interacts with other parts of the body. It’s like a molecular fingerprint, ensuring it does its intended job.
Imagine the body as a giant construction site. Steroids are like specialized tools. Some are wrenches, some are screwdrivers, and some are even tiny cranes! Each functional group is like the handle or the tip of that tool, designed for a specific task.
These functional groups are what allow steroids to act as signals. They tell cells what to do, when to grow, or when to stop. It’s a silent language spoken by molecules all over our bodies. It’s a constant conversation happening at the smallest level.
Sometimes, we see steroids with even more complex functional groups attached. These could be esters, which involve a carbonyl group and an oxygen. Or ethers, where oxygen connects two carbon chains. These are like fancier attachments for our molecular tools.
The variety is truly astounding! From the cholesterol that builds our cell walls to the hormones that shape our development, steroids are everywhere. And it’s those highlighted functional groups that are the secret sauce behind their incredible power and diversity.
So, the next time you hear about steroids, remember that it’s not just one thing. It’s a whole family of molecules. And the magic lies in those little highlighted bits – the hydroxyls, the ketones, the methyls, and more. They are the architects of our biological world!
It’s like being a detective, but instead of solving crimes, you’re uncovering the secrets of life itself. And the clues are these fascinating functional groups. They are the key to understanding how these molecules perform their vital roles. It’s a wonderfully intricate puzzle.
The sheer elegance of it all is breathtaking. Nature has found such a simple yet effective way to build such complex and vital molecules. And by highlighting these functional groups, we're getting a glimpse into that ingenious design. It’s a constant source of wonder.
So, keep your eyes peeled for those -OHs and C=Os! They’re not just random letters and symbols. They are the energetic bits that make steroids tick. They are the unsung heroes of our biology. They are what make these molecules so darn interesting and essential!
It's like discovering a hidden language spoken by our own bodies. And the key to understanding it lies in these small but mighty chemical groups!
Learning about these functional groups is like getting a backstage pass to the molecular world. You start to see the patterns, the logic, and the sheer brilliance of biological design. It’s a journey of discovery that’s both educational and utterly captivating.
And the best part? You can start to spot them yourself! Once you know what to look for, you’ll see these functional groups popping up everywhere. They are the building blocks, the connectors, and the activators of life. They are truly the stars of the show in the incredible world of steroids.
