Identify The Generic Outer Electron Configuration For The Halogens
So, I was rummaging through my attic the other day, you know, the place where forgotten dreams and slightly dusty board games go to retire. I found this old chemistry textbook from my high school days. Flipping through it, I landed on a chapter about the periodic table. Suddenly, a wave of nostalgia hit me. Remember those endless rows of elements, each with its own weird symbol and atomic number? It felt like a secret code, and I remember thinking, "How on earth do you even begin to crack this thing?"
But then, I stumbled upon the halogens. Fluorine, chlorine, bromine, iodine, astatine... and that mysterious, synthetic one, tennessine. They just seemed so... intense. Always craving something, always ready to react. It was like they had this inherent itch they just had to scratch. And it got me thinking, what's their secret? What makes them tick? Is there some universal truth about these elements that ties them all together, despite their different personalities?
Turns out, there totally is! And it’s all about something super fundamental in chemistry: their outer electron configuration. Yeah, I know, electron configurations sound like something out of a sci-fi novel, but trust me, they're the building blocks of everything. Think of it like this: imagine each element is a person, and their electron configuration is their wardrobe. Some people have a closet bursting with every outfit imaginable, ready for any occasion. Others? Well, they’re a bit more… minimalist. And the halogens? They’re the ones who always seem to be missing that one perfect accessory to complete their look. It's a real cliffhanger, and that’s what makes them so fascinating.
We’re going to dive deep into this, but don't worry, no need to wear a lab coat. We're keeping it casual, like we're just chatting over coffee about why some people just love to talk about the weather, while others are all about the latest gossip. It’s the same vibe, just with atoms instead of humans.
The Halogen Hotness: What's Their Deal?
Let's start with the name itself. "Halogen" comes from Greek, meaning "salt-former." And boy, do they live up to that name! They’re practically BFFs with metals, readily pairing up to create all sorts of salts we use every day. Think of common table salt, sodium chloride (NaCl). That chlorine? One of our halogen buddies!
But why are they so eager to team up? It’s all about achieving a state of blissful stability. Atoms, much like us, want to be comfortable and content. And in the atomic world, comfort means having a full outer electron shell. It's like having all your ducks in a row, your ducks being those pesky electrons.
Now, the periodic table is arranged in a way that’s super helpful. Elements in the same column, or group, tend to have similar chemical properties. And guess what? The halogens all hang out in Group 17. Coincidence? I think not! This grouping is a big clue that they share something fundamental in their atomic makeup, something that dictates their behavior. And that "something" is, you guessed it, their outer electron configuration.
Peeking Inside the Atomic Closet: Electron Configurations
Okay, time for a quick refresher, or maybe a first-time introduction, to electron configurations. Think of an atom’s electrons as tiny little things orbiting the nucleus. They don't just whiz around randomly, though. They occupy specific energy levels, kind of like floors in a building, and within those levels, they exist in different shapes called orbitals, like different rooms on each floor. The outermost shell is the one that matters most when it comes to chemical reactions. It's the "valence shell," and the electrons in it are the "valence electrons." These are the ones getting all the attention!
The arrangement of these valence electrons determines how an atom will interact with others. It's like looking at someone's outfit and knowing if they're ready for a casual hangout or a formal gala. Different outfits, different intentions, right?
So, what's the deal with the halogens? What’s their standard outfit look like? Let's break it down.
The Noble Gas Envy: A Quest for Fullness
The ultimate goal for most atoms is to achieve the electron configuration of the noble gases. These guys, lounging at the very end of the periodic table (Group 18), are the epitome of atomic chill. They’re notoriously unreactive because their outermost electron shells are completely full. They've got it made, the atomic equivalent of a permanent vacation.
Imagine you’re at a party, and everyone else is mingling, swapping stories, maybe even doing a bit of polite gossiping. And then there’s the noble gas, just chilling in a corner, perfectly content, not needing to talk to anyone. That's the vibe. They've got their full set of electrons, and they're not looking to share or borrow.
Now, the halogens? They’re so close to this perfect state. They’re like the party guest who’s almost got the perfect conversation starter but is missing just one word. They have almost a full outer shell, but not quite. It's this tantalizing "almost there" situation that drives their reactivity.

The Generic Outer Electron Configuration of Halogens: The Big Reveal!
So, after all that build-up, what is the magic formula? What’s the common thread that ties Fluorine (F) to Iodine (I) and everyone in between?
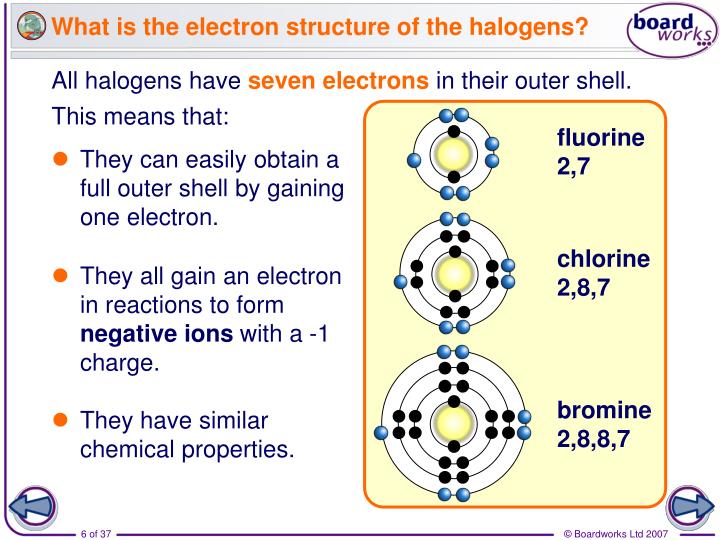
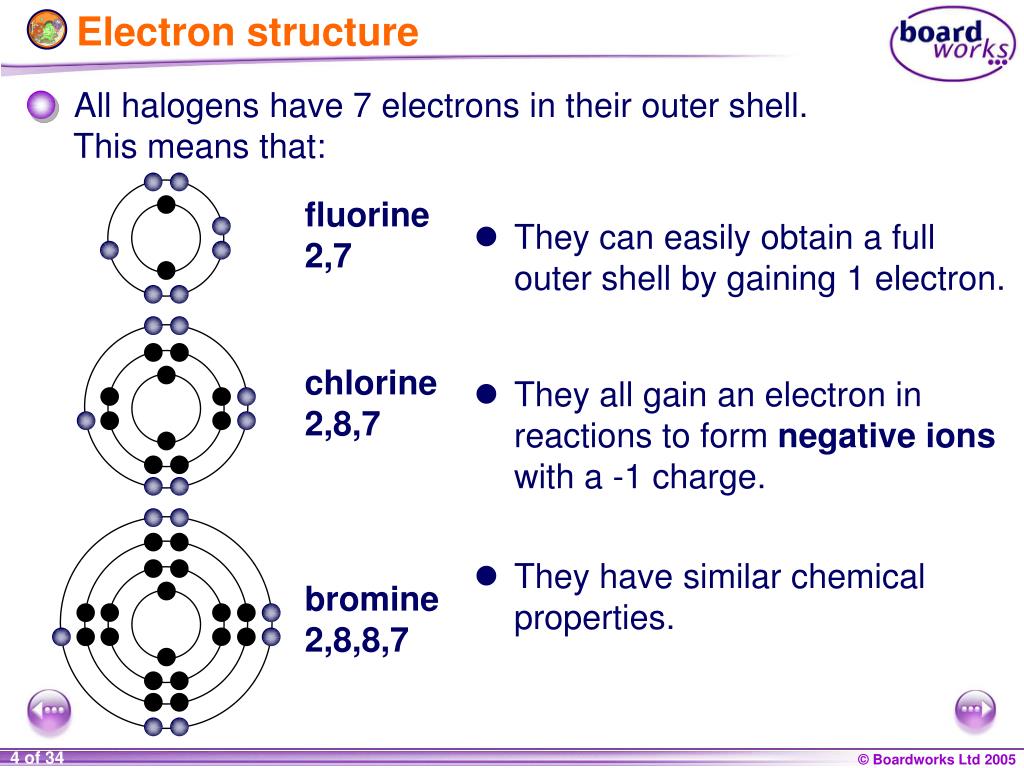
The generic outer electron configuration for the halogens is: ns²np⁵.
Let’s unpack that, shall we? It’s not as scary as it looks!
- 'n': This represents the principal energy level, or the "floor" in our building analogy. For halogens, this number changes depending on the specific element. For example, fluorine is in the second period, so its 'n' would be 2. Chlorine is in the third period, so its 'n' would be 3, and so on.
- 's²': This part means there are two electrons in the 's' subshell of that energy level. Think of the 's' subshell as a small, spherical room. It can hold a maximum of two electrons, and the halogens have filled it up completely. They've got both of those spots taken.
- 'np⁵': This is the crucial part. It means there are five electrons in the 'p' subshell of that same energy level. The 'p' subshell is shaped a bit differently and can hold a maximum of six electrons.
So, when you put it all together, a halogen atom has seven electrons in its outermost shell (2 in the 's' subshell + 5 in the 'p' subshell). Seven is so close to the magic number eight, which is what the noble gases have!
This is why they’re so eager to gain one more electron. If they can just snag that one missing electron, they'll achieve that coveted noble gas configuration, filling their outer shell and becoming stable and unreactive. It's the ultimate chemical glow-up!
Why This Configuration Matters: The Reactivity Connection
This ns²np⁵ configuration is the secret sauce behind the halogens' notorious reactivity. They are hungry for that one electron. This hunger drives them to:

- Gain an electron: This is their favorite pastime. When they react with metals, for example, the metal readily gives up an electron (or more, depending on the metal), and the halogen happily snatches it up. Boom! A stable salt is formed. It's a win-win, or more accurately, a win-gain.
- Form covalent bonds: They can also share electrons with other non-metals. In this case, two halogen atoms can come together and share their unpaired electrons to effectively "borrow" from each other, creating a stable diatomic molecule (like Cl₂, Br₂, I₂). It's like a shared custody agreement for electrons, ensuring everyone feels like they have a full set.
- Be highly electronegative: Electronegativity is a measure of how strongly an atom attracts electrons in a chemical bond. Because they’re so close to achieving a full shell, halogens are extremely electronegative. They’re the ultimate electron hoarders!
Think about it: if you were almost done with a really challenging puzzle, wouldn't you be super focused on finding that last piece? That's the halogen mindset. They have this intense drive to complete their outer shell.
Examples in the Wild (and in Your Kitchen!)
Let's look at a couple of examples to make this concrete:
Chlorine (Cl): Chlorine is in the third period, so its 'n' is 3. Its outer electron configuration is 3s²3p⁵. It has 7 valence electrons. When it reacts with sodium (Na), which has one valence electron (3s¹), sodium gives its electron to chlorine. Sodium becomes Na⁺ (with a full outer shell like neon), and chlorine becomes Cl⁻ (with a full outer shell like argon). Together, they form NaCl – table salt!
Bromine (Br): Bromine is in the fourth period, so 'n' is 4. Its outer configuration is 4s²4p⁵. Again, 7 valence electrons, that familiar pattern. Bromine also loves to grab an extra electron to achieve the stable configuration of krypton.
This pattern repeats for every halogen. It's the universe's way of saying, "Hey, these guys are related, and here's why!"
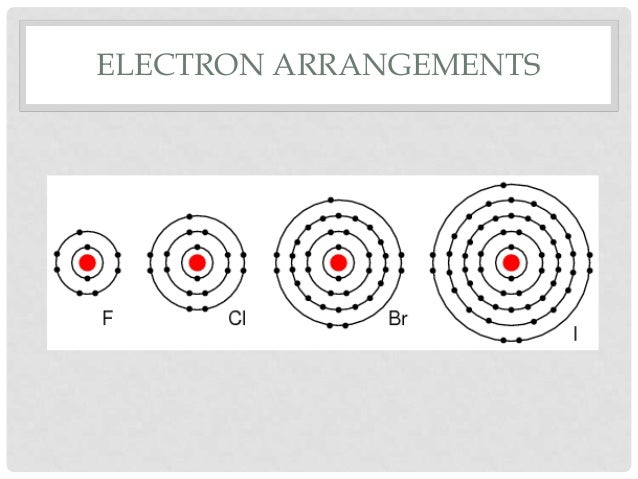
Beyond the Configuration: The Impact of Atomic Size
While the electron configuration is the primary driver, it’s worth mentioning that atomic size also plays a role in the subtle differences between halogens. As you go down the group (from fluorine to astatine), the atoms get bigger. This means the outermost electrons are further from the nucleus and are shielded by more inner electrons. This slight variation can affect things like electronegativity and reactivity, but the fundamental ns²np⁵ configuration remains the unifying theme.
It's like saying that while all superheroes wear capes, some have silk capes, and others have rugged leather ones. The core concept is the same, but the material adds a little flavor.
The Halogen Legacy: Essential and Everywhere
So, next time you sprinkle salt on your fries, or use toothpaste with fluoride, or even marvel at the vibrant colors of iodine crystals (carefully, of course!), remember the humble halogens and their quest for that last electron. Their seemingly simple outer electron configuration, ns²np⁵, is a powerful testament to the underlying order and predictability of chemistry.
It's a reminder that even the most reactive and seemingly complex elements have a fundamental, elegant structure that explains their behavior. It’s the atomic equivalent of a really good plot twist – everything makes sense once you know the secret!
This basic understanding of electron configuration is what allows chemists to predict how elements will behave, design new materials, and understand the very world around us. It's not just about memorizing symbols and numbers; it's about understanding the language of atoms, and the halogens are definitely fluent speakers in that language. They’re always ready to have a conversation, or, more accurately, to snatch up an electron!
Pretty neat, huh? It’s like finding out your favorite celebrity’s secret talent is actually something incredibly mundane but also foundational. Who knew that a few electrons in a specific arrangement could lead to so much chemical drama and utility?
