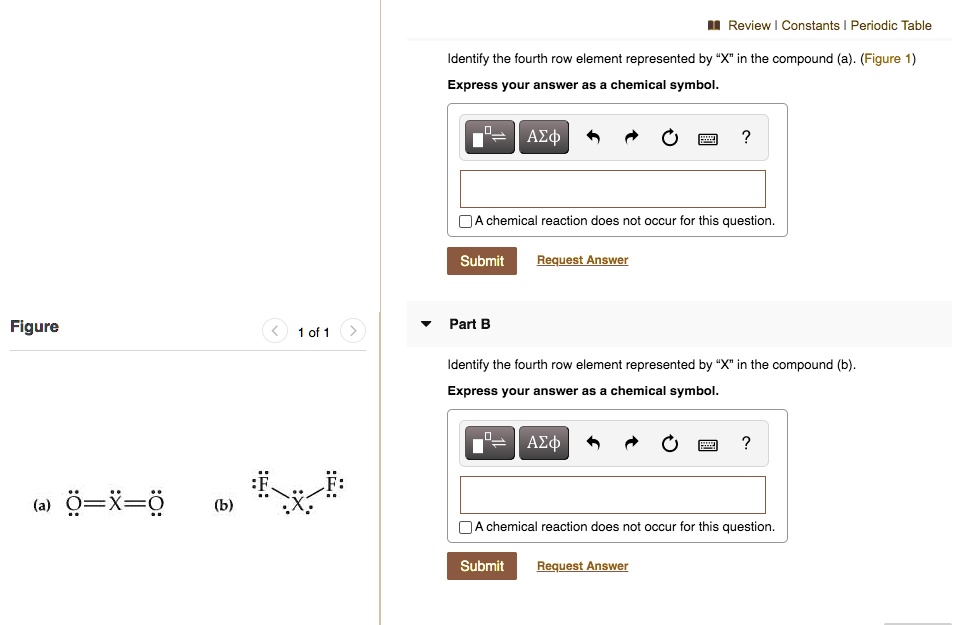
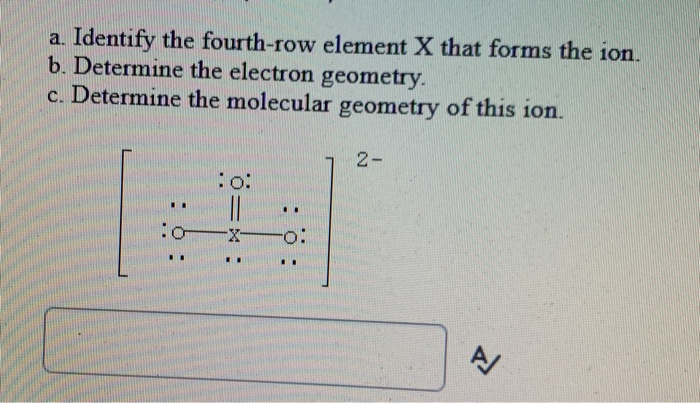
Identify The Fourth-row Elements X That Form The Following Compounds
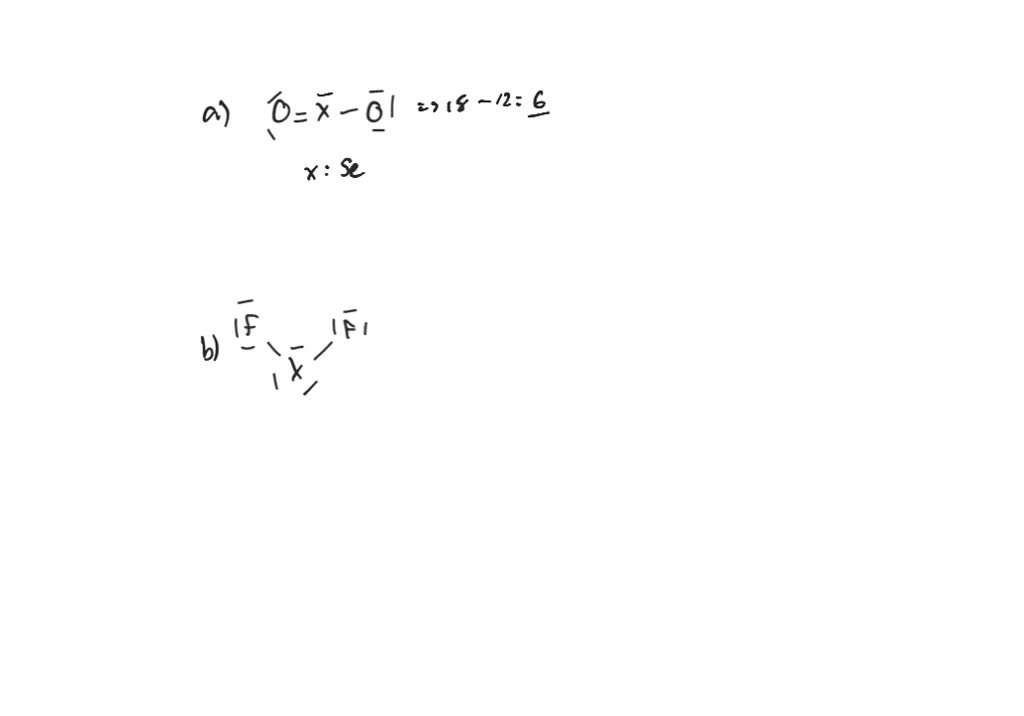
Get ready for a cosmic adventure into the heart of matter! We're about to become super-sleuths, piecing together clues from the wacky world of chemistry to uncover some seriously cool fourth-row elements. Think of it like a treasure hunt, but instead of gold doubloons, we're digging for building blocks of the universe!
Imagine elements as tiny Lego bricks that make up absolutely everything you see, touch, and even think about. Some are common as dirt, while others are rare and exciting. Today, we're focusing on a special crew from the fourth row of the periodic table – a row packed with personality and power!
Our mission? To identify the mysterious "Element X" by looking at the fun (and sometimes fancy!) compounds it forms. These compounds are like little chemical friendships, where Element X teams up with other elements to create brand new substances with all sorts of amazing properties. It's like knowing your friend likes pizza and then figuring out they're the one ordering it for the party!
The Case of the Colorful Chloride!
Our first suspect is X, who loves to hang out with chlorine. Not just any old chlorine, mind you, but the kind that forms XCl₃. This little molecule is quite the show-off, often appearing as a pale yellow solid that melts at a surprisingly low temperature. Think of it as a chemical chameleon, easily transforming between states!
Now, this pale yellow friend isn't exactly your everyday kitchen staple. It's more of a specialty ingredient, used in some neat industrial processes. Imagine needing a special glue that only works under specific conditions – that's the kind of vibe XCl₃ gives off. It's not meant for making cookies, but it's definitely important for certain high-tech operations!
The "Cl" part is, of course, our good ol' chlorine. It's a reactive fellow, always looking to make bonds. But it's Element X that gives this compound its unique characteristics. We're looking for a fourth-row element that plays nicely with chlorine to create this specific trio of atoms.
The Sparkly Sulfide Saga!
Next up, Element X is showing off its sparkly side, forming X₂S. This compound is less about pale yellow and more about... well, not much color at all, often appearing as a colorless solid. But don't let its subtle appearance fool you! This is a compound that often hints at some rather electrifying capabilities.
Think of the times you've seen something glimmer or shine. Sometimes, it's not just light playing tricks; it's the very structure of the material. X₂S is a quiet achiever, a foundational piece for some seriously impressive electronic gadgets. It's like the unsung hero behind your favorite video game console or that super-fast internet connection!
The "S" here stands for our friendly neighborhood sulfur. Sulfur is another element that loves to bond, and when it teams up with our mystery element X in a two-to-one ratio, something quite special happens. This particular pairing is a hallmark, a chemical fingerprint that points us towards our elusive fourth-row resident.
The Fiery Fluoride Fiasco!
Our Element X is really getting into the swing of things, now forming XF₄. This compound is a bit of a dramatic performer. It’s a colorless gas at room temperature, which means it’s practically invisible, but it’s also incredibly reactive. Like a secret agent, it can be tricky to pin down!
Imagine a gas so eager to react that it can change other substances on contact. That's the power packed into XF₄! It's not something you'd find in your salad dressing, but it's a crucial player in processes that make things like certain plastics super strong and resilient. It's the secret ingredient that makes things tough and durable!
The "F" in this equation is our super-electronegative friend, fluorine. Fluorine is like the energetic cheerleader of the periodic table, always ready to grab electrons and form bonds. When it forms this four-to-one relationship with our Element X, it’s a strong indicator of X's particular chemical talents. This specific combination is a big clue!
The Oxygen's Oxide Opinion!
And finally, the grand finale! Element X teams up with the ever-present oxygen to form XO₂. This is a compound we see in various forms, sometimes as a solid, and it's known for being a pretty good oxidizing agent. Think of it as a catalyst for change, helping other reactions happen smoothly.

This compound, XO₂, is like the reliable workhorse of the chemical world. It's involved in processes that are vital for everything from making certain metals stronger to helping in the creation of some pharmaceuticals. It's the kind of element that's always there, quietly contributing to important advancements. It’s the dependable sidekick!
Oxygen, the "O," is all around us, and when it pairs up with a fourth-row element to form this specific dioxide, it creates a compound with a very distinct personality. This ratio, this particular partnership, is a super-important piece of evidence in our element-finding mission. It’s the final puzzle piece we need!
The Grand Reveal!
So, let's put all our clues together! We have an element from the fourth row that forms:
- XCl₃ (pale yellow, low melting point)
- X₂S (colorless, hints at electrical properties)
- XF₄ (colorless gas, highly reactive)
- XO₂ (various forms, good oxidizing agent)
Think about the elements in the fourth row. We've got potassium and calcium, which are super reactive metals. Then comes scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc. After that, we have gallium, germanium, arsenic, selenium, bromine, and krypton. That's a lot of characters in our chemical play!
Consider the behaviors we've observed. The ability to form compounds like XCl₃ and XF₄, with these specific formulas, often points towards elements that have a bit of a metallic flair but can also exhibit more complex bonding. The hints of electrical properties in X₂S and the oxidizing nature of XO₂ are also crucial pointers.
Drumroll, please! The element that fits all these fantastic characteristics, the elusive fourth-row Element X, is none other than GERMANIUM! Isn't that exciting? This element, often associated with semiconductors and modern technology, is the mastermind behind all these cool compounds.
Germanium, or Ge, is a true superstar of the fourth row. It’s like the Swiss Army knife of elements, capable of forming so many different types of bonds and compounds. Its journey from a mysterious element to a vital component in our world is a testament to the wonders of chemistry!
So next time you're using a computer or a smartphone, remember the incredible Germanium, diligently working behind the scenes in its various chemical guises. You've just solved a fascinating chemical mystery, and that’s something to be super proud of! Keep exploring the amazing world of elements; there are always more adventures waiting!
