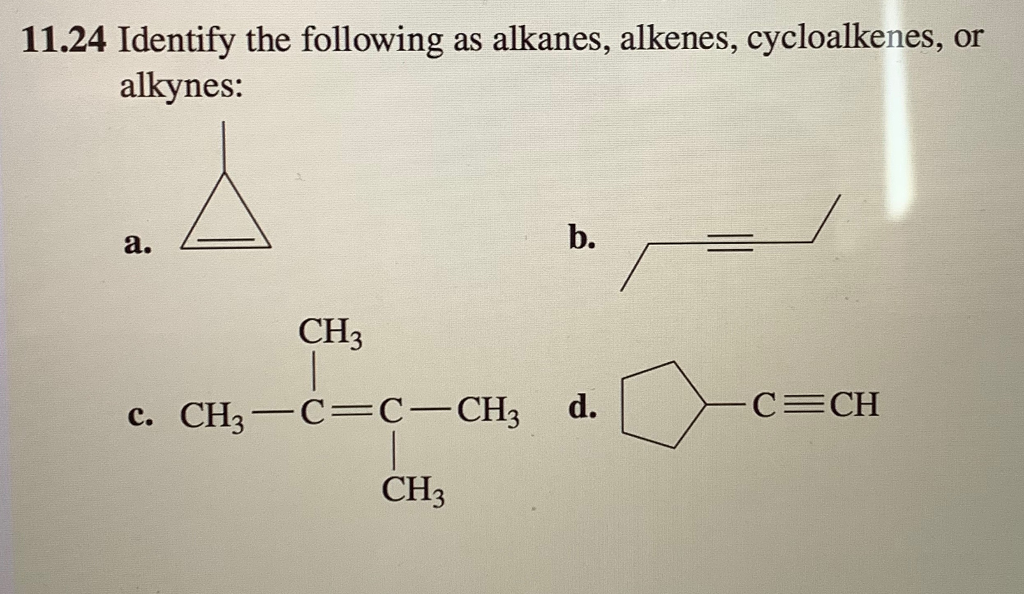
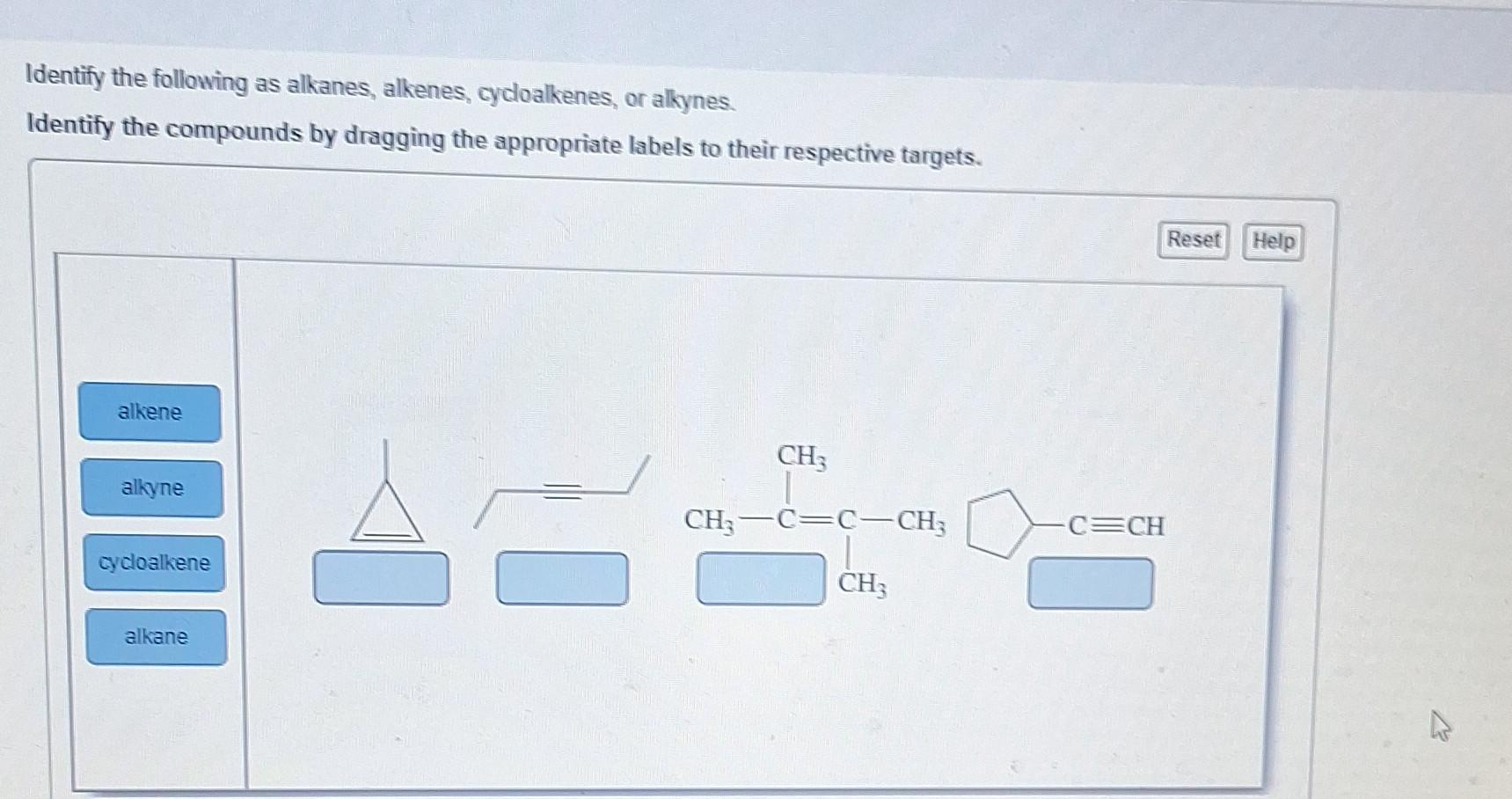
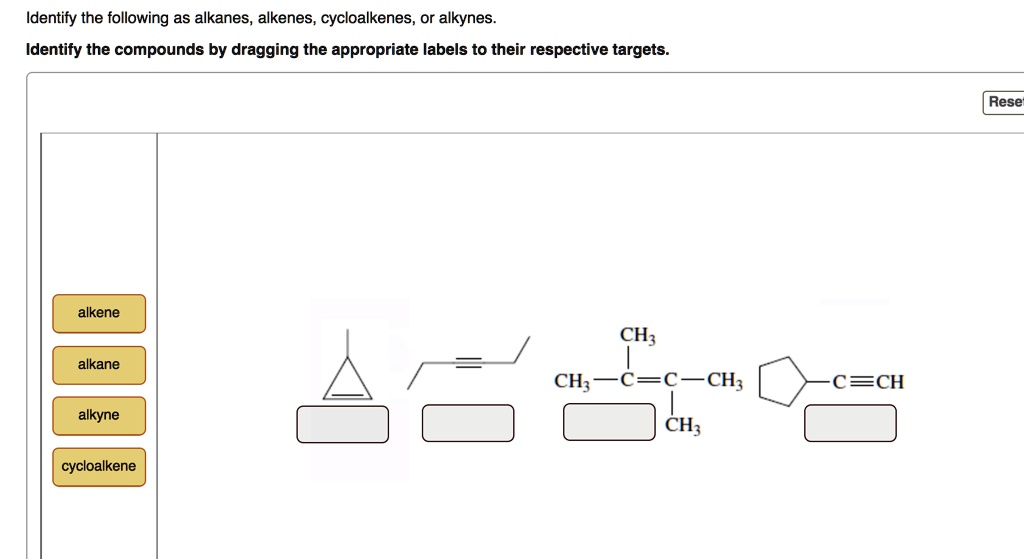
Identify The Following As Alkanes Alkenes Cycloalkenes Or Alkynes

Hey there, science curious pals! Ever glance at some weird chemical names and feel a tiny bit intimidated? Like, what even are these things? Well, let me tell you, it’s not all super-serious lab coats and complicated equations. Sometimes, it's just about figuring out if something's got a chill vibe or if it's ready to party! Today, we’re gonna peek behind the curtain of some basic organic chemistry. Think of it as a fun little game of "Spot the Difference," but with molecules. No stress, just good vibes and maybe a few giggles.
We're diving into the world of hydrocarbons. Fancy word, right? But really, it just means molecules made of hydrogen and carbon. That's it! The building blocks of, well, a LOT of stuff. And we’re going to learn how to tell them apart. It's like being a detective, but your magnifying glass is, uh, your brain. And the "clues" are the little letters and numbers in their names, or even their shapes!
So, let’s get started with our first category. Drumroll, please…
Alkanes: The Chill Ones
Meet the alkanes. These guys are the ultimate laid-back dudes of the hydrocarbon world. They're all about keeping things simple. How do you spot an alkane? Easy peasy. Look for the suffix "-ane" at the end of their name. Think methane, ethane, propane, butane. See the pattern?
Chemically speaking, alkanes are like the introverts of molecules. They're super stable. All their carbon atoms are connected by single bonds. That's it. No double or triple bonds causing a scene. Just nice, cozy single bonds. They're saturated, meaning they've got as many hydrogen atoms as they possibly can. Think of it like a fully packed suitcase – no more room for anything else!
A super quirky fact? The first four alkanes – methane, ethane, propane, and butane – are gases at room temperature. Yep, the stuff we use to grill burgers (propane!) or that's in some lighters (butane!). Methane is also what cows burp out. Seriously. Nature's little gas factories!
And here’s a fun detail: the longer the alkane chain, the more likely it is to be a liquid or even a solid. So, think of your super-long alkanes as the ones who are chilling on the couch, maybe a bit sluggish. They’re the foundation for things like waxes and oils.
Spotting an alkane is like recognizing a friend who always wears the same comfortable sweater. Reliable, predictable, and totally chill. They’re not trying to impress anyone with fancy bonds. They’re just… being alkanes. And that’s pretty cool!
Alkenes: The Party Starters
Now, let’s crank up the energy! Introducing the alkenes. These guys are a bit more exciting. The giveaway here? Their names end in "-ene". Think ethene, propene, butene. They’re the slightly more adventurous cousins of the alkanes.
What makes them more exciting? They’ve got at least one double bond between two carbon atoms. This double bond is like a little spark plug. It makes them way more reactive than alkanes. They’re unsaturated, meaning they can accept more hydrogen atoms if they wanted to, thanks to that double bond. It’s like they’re always ready to add something new to their molecular party.
Here’s a funny thought: that double bond is like a perfectly timed handshake. It’s stronger than a single bond, but also more flexible in how it can interact. This double bond is the key to so many cool reactions. It’s what allows things like plastics to be made!
Fun fact alert! Ethene (or ethylene, if you’re feeling informal) is a plant hormone. It actually helps fruits ripen. So, next time you see a banana turning yellow, you can thank ethene for the party! It's like the backstage manager of the fruit ripening world, subtly nudging things along.
So, if you see an "-ene" ending, you know you’re dealing with a molecule that’s a little more dynamic. They’re not just chilling; they're ready to mingle and react. Think of them as the life of the hydrocarbon party!
Cycloalkenes: The Ring Rockers
Alright, let’s get a little geometric. Welcome to the world of cycloalkenes. These are the alkenes, but with a twist! They’ve decided to form a ring. And guess what? They still have that special ingredient: at least one double bond.
The "cyclo" part of their name tells you they're in a circle. Think cyclobutene or cyclopentene. They're like an alkene that decided to hold hands with itself. This ring structure gives them some unique properties.
So, you have a double bond and a ring. It’s like a car with a spoiler and a sunroof. Pretty cool. The double bond still makes them reactive, but the ring structure can influence how they react. It’s like a party happening inside a very exclusive club.

A quirky detail: the smallest cycloalkene with a double bond is cyclopropene. It's a pretty strained little ring, like trying to cram too many people into a tiny photo booth. It's a bit wobbly but still fun!
These guys are often found in natural products, like some flavors and fragrances. So, that pleasant smell from a flower? Might be a cycloalkene saying hello! They’re not as common as the straight-chain guys, but they add a touch of elegance and complexity to the hydrocarbon family. They're the stylish dancers of the molecule world, gracefully twirling in their rings.
Alkynes: The High-Energy Daredevils
Finally, we arrive at the daredevils of the hydrocarbon family: the alkynes! These are the ones who don't mess around. Their names end in "-yne". Think ethyne (also known as acetylene), propyne, butyne.
What makes them so daring? They’ve got not one, but at least one triple bond between two carbon atoms. A triple bond! That’s like three handshakes happening all at once. It's super strong and super energetic. These guys are the most unsaturated of the bunch, ready to react with anything and everything.
The triple bond is like a tiny rocket engine. It’s packed with energy. This makes alkynes super useful in certain applications. Think welding! Acetylene is used in oxy-acetylene torches because it burns at an incredibly high temperature. Talk about a fiery personality!
Here’s a fun fact: the simplest alkyne, ethyne (acetylene), is a gas that’s highly flammable and explosive. So, definitely not something to play with! It’s the rockstar of the hydrocarbon world – bright, powerful, and a little bit dangerous.
If you see an "-yne" ending, you know you're dealing with a molecule that's all about intensity. They’re the ones who are going to make things happen. They're the ultimate go-getters, always ready to unleash their energy.
So there you have it! Alkanes are the chill, saturated single-bonders. Alkenes are the lively, unsaturated double-bonders, often part of cool reactions. Cycloalkenes are the ring-wearing alkenes, adding a geometric flair. And alkynes are the high-energy, triple-bonded daredevils.
Next time you see a chemical name, take a peek at that ending. Is it "-ane," "-ene," or "-yne"? Is it in a ring? You’ll be identifying these molecular personalities like a pro! It’s a small step, but it unlocks a whole world of understanding. And who knows, maybe you'll start seeing the world of chemistry a little differently – with more curiosity and a lot more fun!
