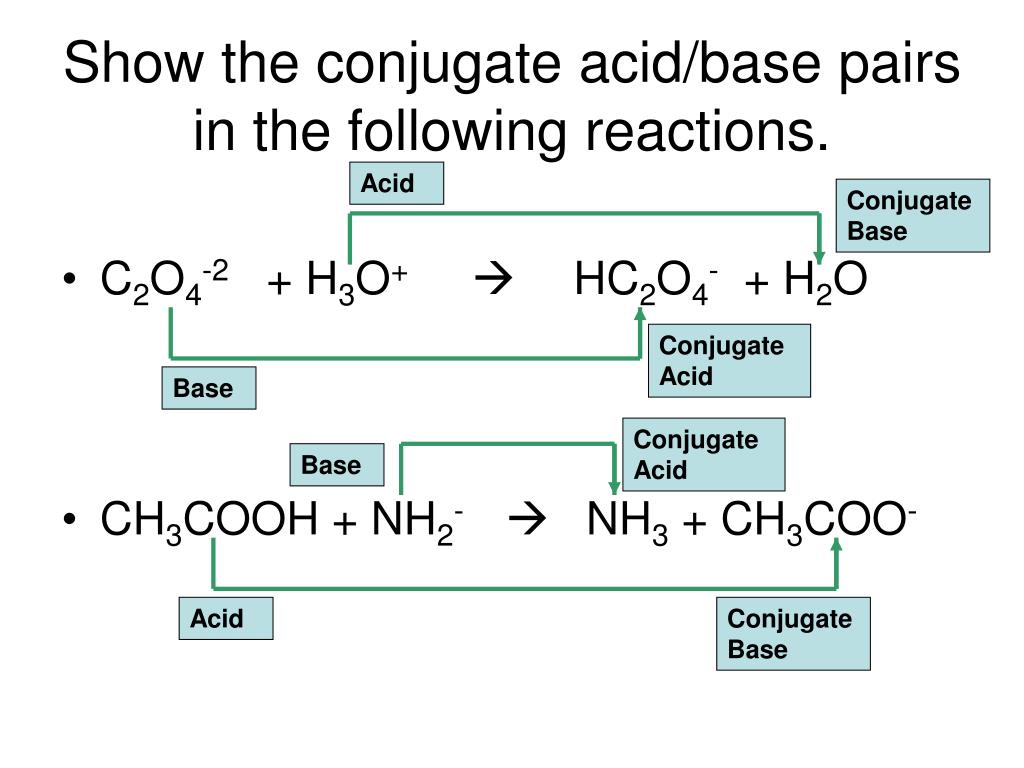
Identify The Conjugate Acid Base Pairs In The Following Reactions

Welcome, budding chemists and curious minds, to a world where molecules play a fascinating game of give and take! Today, we're diving into the super cool concept of conjugate acid-base pairs. Now, you might be thinking, "Acid? Base? Sounds complicated!" But trust me, it's actually a lot like figuring out who's passing what in a friendly game of tag. It's a fundamental idea in chemistry that helps us understand how reactions happen, why some substances behave the way they do, and it's surprisingly useful in everything from making your morning coffee taste just right to developing life-saving medicines.
So, what's the big deal with conjugate acid-base pairs? Think of it as identifying the partners in a chemical dance. In any acid-base reaction, there's always a molecule that donates a proton (a little hydrogen ion, often written as H+) and a molecule that accepts that proton. These two molecules, the proton donor and the proton acceptor, are intimately related – they are each other's conjugate. The pair consists of the acid and its conjugate base, or the base and its conjugate acid. It’s like a family reunion where you see who's related to whom!
Understanding these pairs is incredibly beneficial. It gives us a framework to predict the outcome of acid-base reactions. If you know what the acid and base are at the start, you can confidently identify their partners after the proton exchange. This skill is crucial for:
- Predicting reaction direction: Knowing the strengths of acids and bases helps us understand which way a reaction will favor.
- Understanding pH: Conjugate pairs are central to buffer solutions, which are vital for maintaining stable pH levels in biological systems (like your blood!) and chemical processes.
- Troubleshooting experiments: If a reaction isn't going as planned, identifying the conjugate pairs can shed light on why.
- Everyday life: From the tanginess of your yogurt to the effectiveness of antacids, conjugate acid-base pairs are at play.
Let's get to the fun part: identifying these pairs in action! We'll look at a few reactions and pinpoint the partners. Remember, the key is to spot the proton (H+) moving from one species to another. The species that loses the H+ becomes its conjugate base, and the species that *gains the H+ becomes its conjugate acid.
Reaction 1: The Classic
HCl (aq) + H₂O (l) ⇌ Cl⁻ (aq) + H₃O⁺ (aq)
In this reaction, we see hydrochloric acid (HCl) acting as the proton donor. It generously gives away its proton to water (H₂O). So, what happens to HCl after it loses its proton? It becomes chloride ion (Cl⁻). Therefore, HCl is the acid, and Cl⁻ is its conjugate base. Now, what about water? It accepted the proton, transforming into hydronium ion (H₃O⁺). Since H₂O gained a proton to become H₃O⁺, H₂O acts as the base in this instance, and H₃O⁺ is its conjugate acid. See? A perfect pair!
Reaction 2: A Slightly Different Flavor
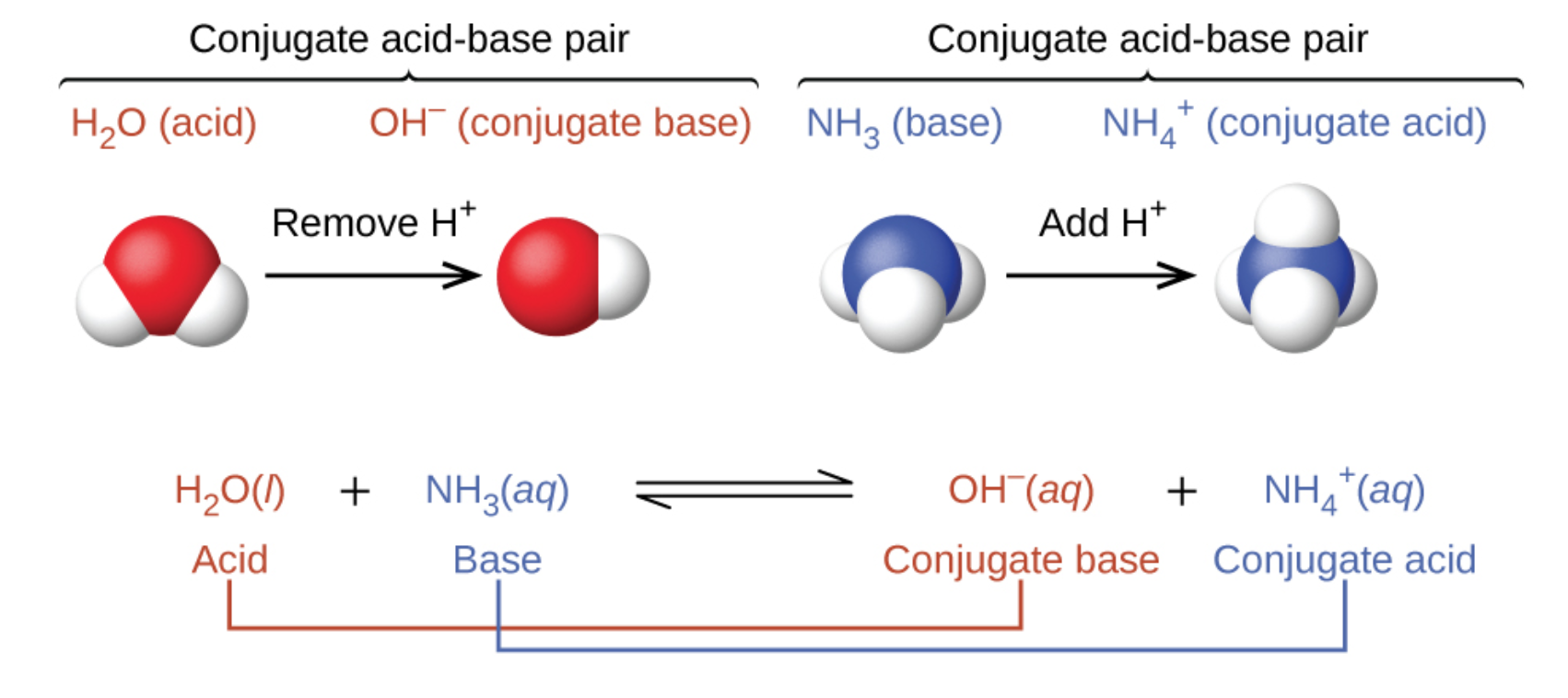
NH₃ (aq) + H₂O (l) ⇌ NH₄⁺ (aq) + OH⁻ (aq)
Here, we have ammonia (NH₃). This time, water (H₂O) is the one donating a proton! Ammonia, being a bit of a proton magnet, readily accepts it. So, when NH₃ accepts a proton, it becomes ammonium ion (NH₄⁺). This means NH₃ is the base, and NH₄⁺ is its conjugate acid. And what does water do? It loses a proton to become hydroxide ion (OH⁻). Therefore, H₂O is the acid in this scenario, and OH⁻ is its conjugate base. Notice how water can act as both an acid and a base? That makes it pretty special and is why we call it amphoteric!
Reaction 3: The Carbonate Caper
CO₃²⁻ (aq) + H₂O (l) ⇌ HCO₃⁻ (aq) + OH⁻ (aq)
Let's look at the carbonate ion (CO₃²⁻). In this reaction, it's the one receiving a proton from water (H₂O). After accepting the proton, CO₃²⁻ transforms into the bicarbonate ion (HCO₃⁻). So, CO₃²⁻ is the base, and HCO₃⁻ is its conjugate acid. And as we saw before, water (H₂O) acts as the acid, donating a proton and becoming the hydroxide ion (OH⁻), which is its conjugate base.
Identifying these pairs might seem like a simple exercise, but it unlocks a deeper understanding of chemical behavior. It’s like learning the secret handshake of acid-base chemistry! Keep practicing, and soon you’ll be spotting these conjugate pairs with ease, impressing your friends and maybe even your chemistry teacher. The world of chemistry is full of these little connections, and understanding conjugate acid-base pairs is a fantastic step into that exciting realm!
