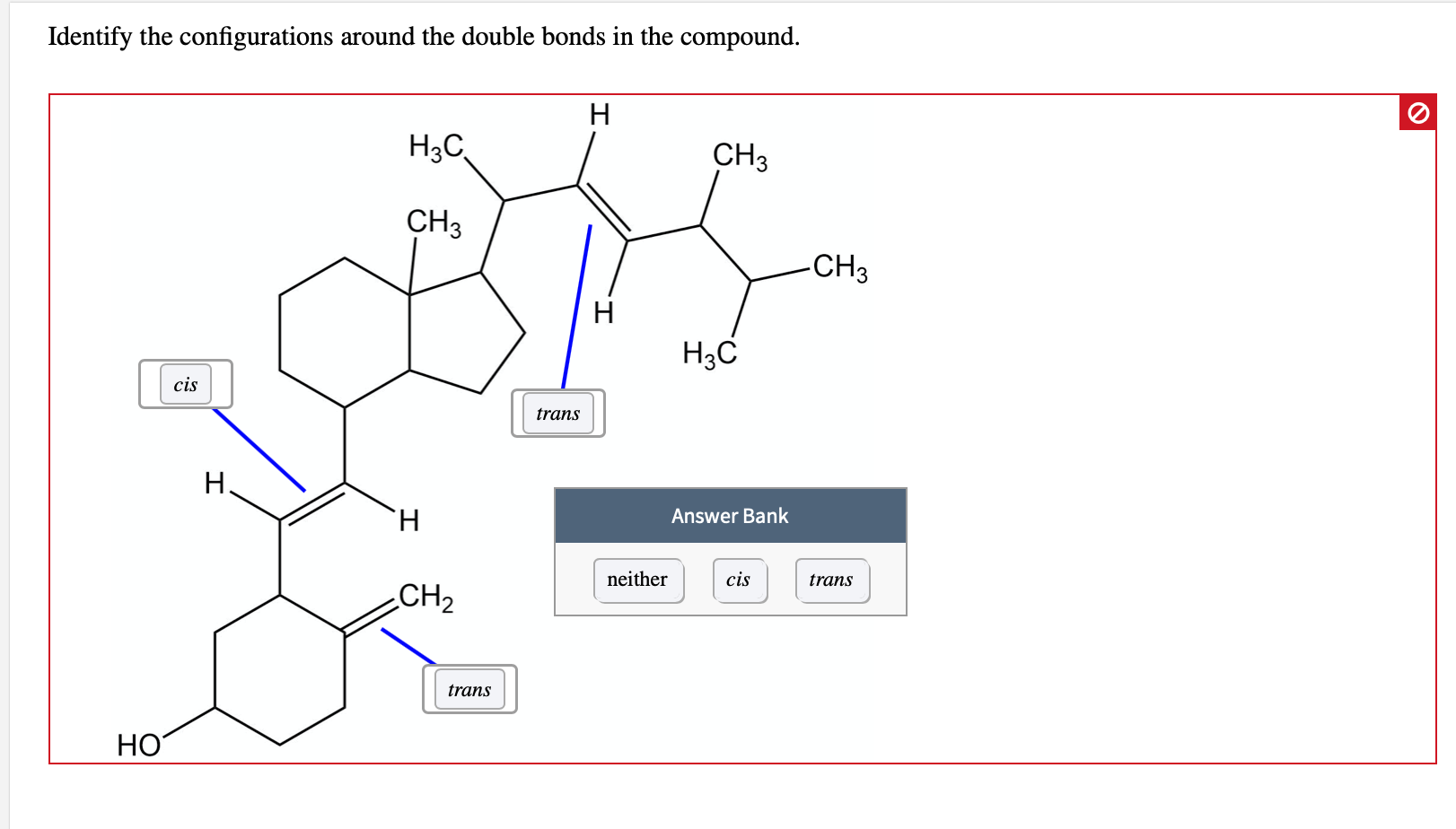
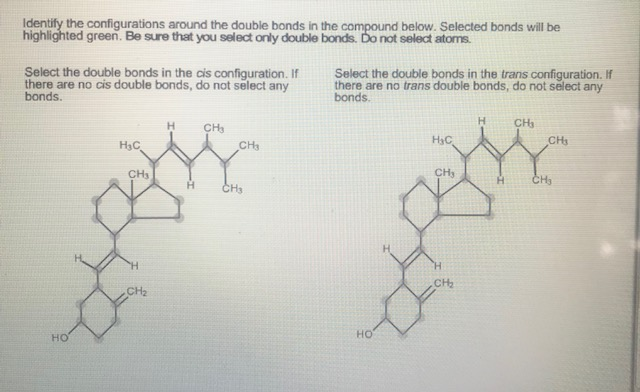
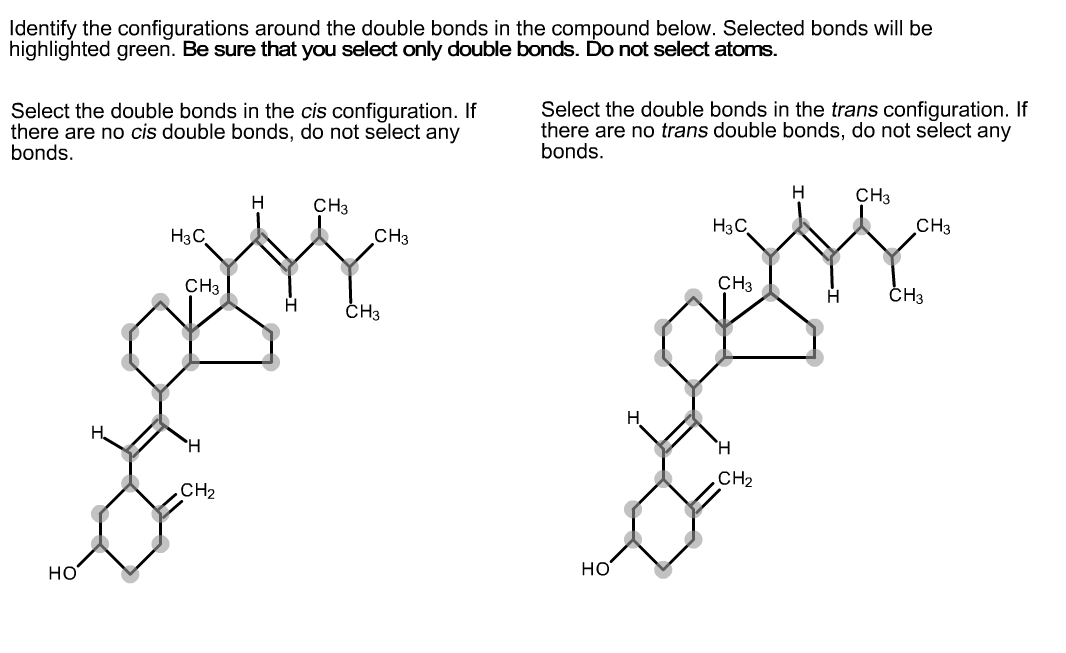
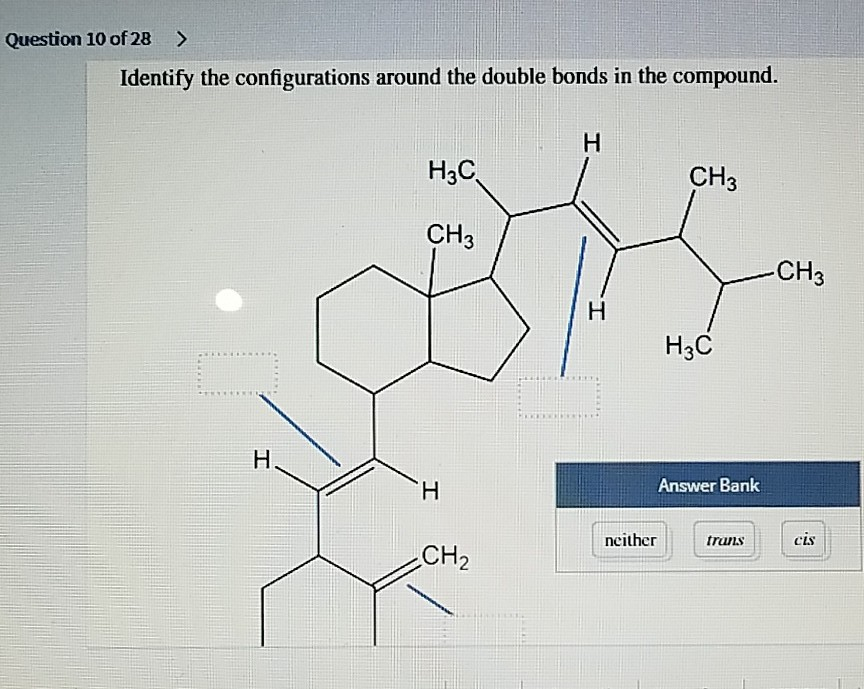
Identify The Configurations Around The Double Bonds In The Compound.
Ever stared at a molecule and felt like you needed a secret decoder ring? Yeah, me too. We’re talking about those fancy configurations around double bonds. It sounds super serious, like something a lab coat enthusiast would whisper about. But honestly, it’s just a little dance of atoms. Think of it like a very polite party. Everyone has their assigned spot, and they’re trying not to bump into each other too much. And, surprise! Some parties are way more organized than others.
So, we’ve got this compound, and it’s got some double bonds. Now, these double bonds are a bit like those awkward family gatherings. They’re the center of attention, and what happens around them is kind of important. But instead of Uncle Barry telling that same old joke, we’ve got groups of atoms hanging out. And their arrangement matters. It’s like deciding who sits next to whom at Thanksgiving. Some arrangements just feel… better. More stable. Less likely to cause a molecular meltdown.
Let’s dive into this. Imagine a double bond as a handshake between two atoms. They’re holding hands pretty tightly. Now, around these holding hands, we have other atoms or groups of atoms. They can be on the same side, or they can be on opposite sides. It’s like having friends standing on either side of the handshake. Are they all clustered together on one side, looking a bit cramped? Or are they spread out, giving everyone a little breathing room?
When these groups are on the same side, we give it a special name. It’s called the Z configuration. Now, why 'Z'? Some say it's for the German word zusammen, which means "together." Makes sense, right? They’re all hanging out together. It’s like the group selfie where everyone’s squished in, grinning. It’s cozy. It’s maybe a little chaotic, but it’s definitely a togetherness kind of vibe. You can picture them all leaning in, whispering secrets.
Then, we have the other option. When these groups are on opposite sides, it’s like they’ve decided to give each other some space. This one gets the name E configuration. Again, there’s a German word involved: entgegen, meaning "opposite." So, they’re literally opposite. Think of it as the polite nod across a crowded room. They acknowledge each other, but they’re not in each other’s personal space. This configuration often feels a bit more chill, a bit more relaxed. It’s the molecular equivalent of a sigh of relief.
Now, here’s the slightly mind-bendy part, and this is where my unpopular opinion really kicks in. Some people get all worked up about which is better. Is E always more stable? Is Z somehow inherently less… elegant? I say, poppycock! It’s all about context. It’s like saying blue is a better color than green. They’re just different! Both configurations have their own charm, their own way of existing. They’re just different flavors of molecular arrangement.
Think about it. A molecule is just trying to find its happy place. The Z configuration is like a tight-knit family huddle. It’s all about closeness. The E configuration is more like a group of friends enjoying a picnic, with plenty of space between their blankets. Neither is inherently superior. They’re just different ways of arranging the same essential components. It’s like choosing between a warm hug and a friendly wave. Both are valid forms of interaction!
So, when you see a compound and you’re tasked with identifying these configurations, don’t get overwhelmed. Just look at those double bonds. See where the groups are hanging out. Are they buddy-buddy on the same side (hello, Z!)? Or are they playing a game of polite distance on opposite sides (welcome, E!)? It’s really that simple. You don’t need to overthink it. You don’t need to judge. Just observe.
Sometimes, the E configuration might be a bit more stable. It’s like having more personal space makes you feel more at ease, less prone to arguments. But sometimes, that closeness of the Z configuration can lead to some really interesting chemical reactions. It’s all about what the molecule is trying to achieve. It’s not about good or bad, it’s about function and form.
It’s like having two different outfits for the same event. One is a bit more relaxed and casual, the other is a bit more formal. You wouldn’t say one is wrong, would you? You’d just say it suits the occasion differently. And that, my friends, is the beauty of these configurations. They’re just different ways for molecules to be themselves. So next time you’re faced with a double bond, just have a little chuckle. It’s not rocket science. It’s just some atoms deciding whether to hold hands or give each other a bit of space. And honestly, that's kind of adorable.
It’s easy to get bogged down in the jargon, to feel like you need a PhD to understand simple things. But these configurations, Z and E, are just labels for the way atoms are positioned. They’re descriptive, not judgmental. They tell a story about the molecule’s shape. And every shape has its own unique beauty and purpose. So, embrace the Z. Embrace the E. They’re both just doing their thing, and that’s perfectly fine. Maybe even a little bit wonderful, in their own molecular way.
Honestly, sometimes I think the most complex part is remembering which German word means what. But once you get the gist, it’s like a secret handshake you can do with chemistry. Just look, point, and say, “Ah, they’re together!” or “Yup, opposite sides, classic!”
So, there you have it. The thrilling, the enigmatic, the… well, the surprisingly straightforward world of double bond configurations. Don’t let the fancy names fool you. It's just about where things are. And that, I think, is something we can all appreciate. A little bit of order, a little bit of personality, all wrapped up in a chemical bond. What’s not to love?
