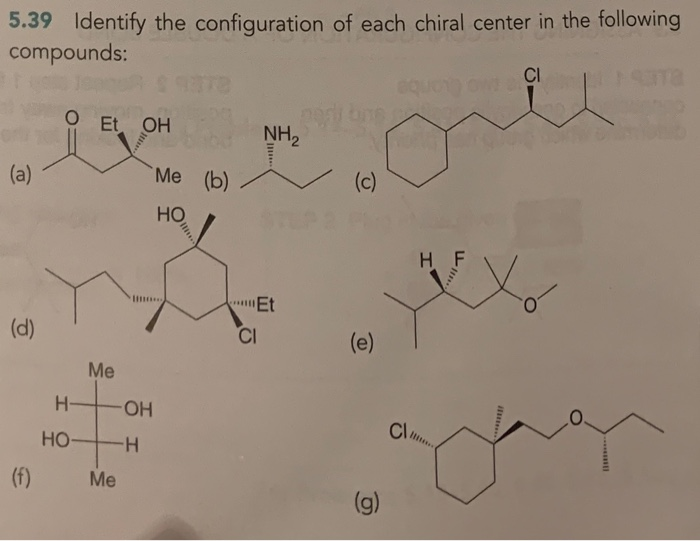
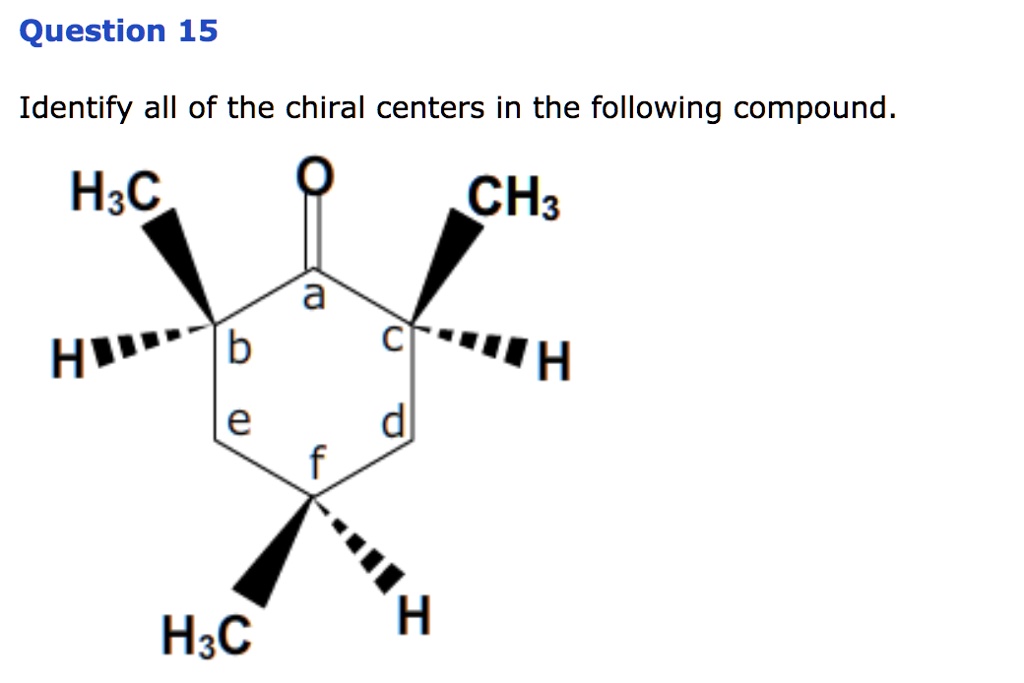
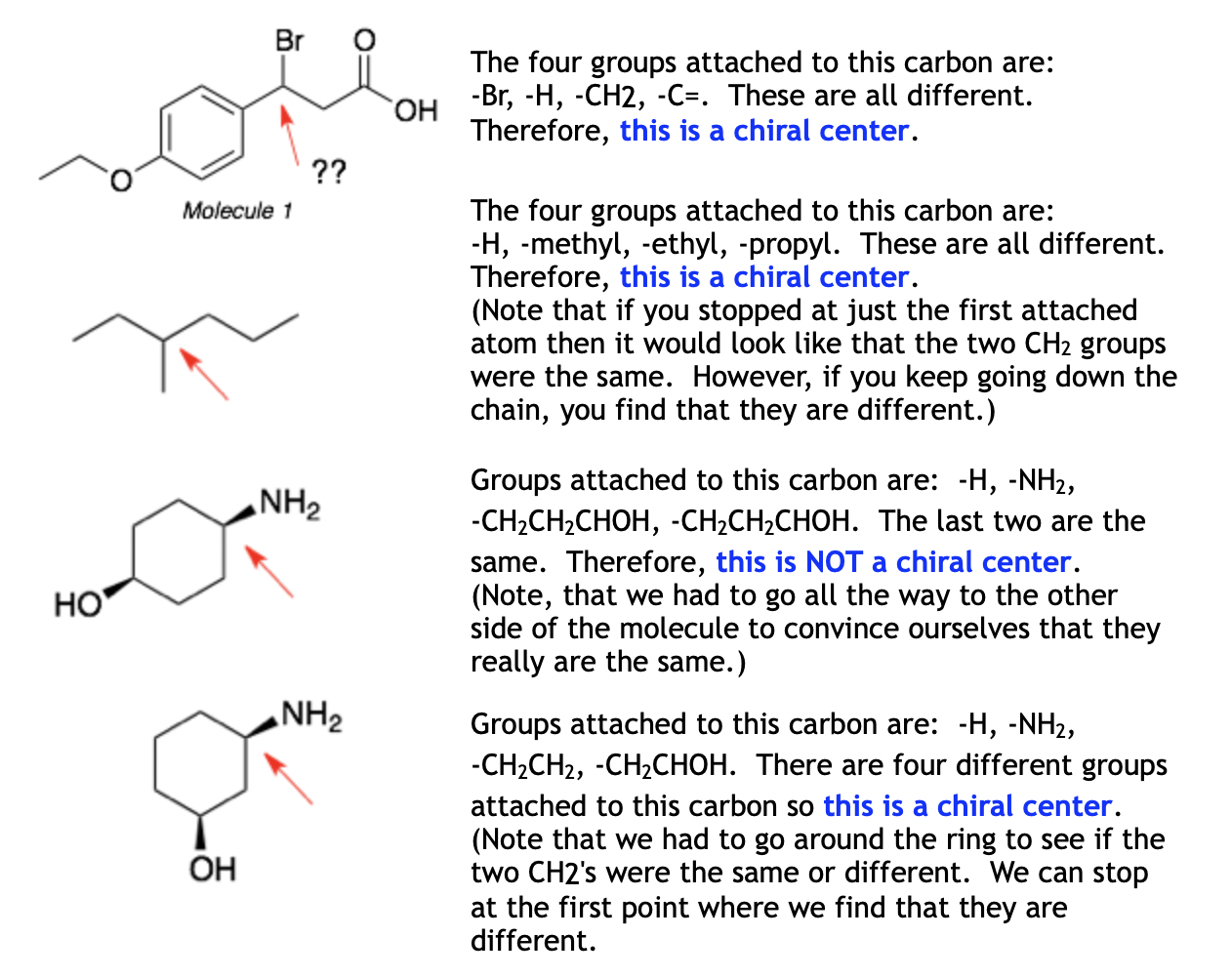
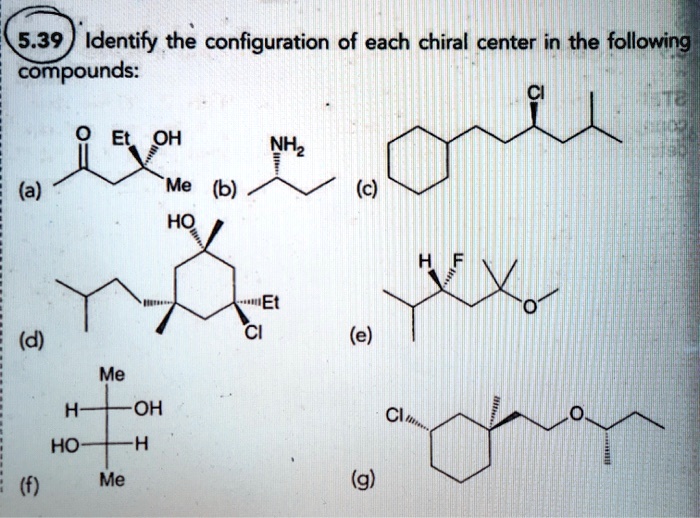
Identify All Chiral Centers In Each Of The Following Compounds:

You know, sometimes I feel like I'm living in a slightly altered reality. Not like, a full-blown Matrix situation, but more like… things just don't quite line up perfectly. I was trying to bake this fancy cake the other day, following a recipe to the letter. But when it came out, it looked a bit… lopsided. One side was higher, the frosting seemed to be sliding off the other. My immediate thought was, "Did I accidentally use the left-handed whisk?"
And then it hit me. This whole "lopsided cake" thing actually has a really cool scientific parallel. It’s all about something called chirality, and in chemistry, it's a HUGE deal. Basically, just like my cake decided to be a bit… different, some molecules can exist in two forms that are mirror images of each other but aren't exactly the same. Think of your hands, right? They're mirror images, but you can't put your right glove on your left hand, can you? It just feels wrong. Well, in the molecular world, this "wrongness" can have some pretty serious consequences.
So, today, we're going on a little adventure to hunt down these chiral centers. Don't worry, it's not going to be a terrifying expedition into the unknown. We're going to be like molecular detectives, sniffing out those special atoms that are responsible for this whole mirror-image business. Think of it as a fun brain teaser, but with the potential to explain why certain drugs work better than others, or why one form of a molecule might taste like sugar and another like metal! Pretty neat, huh?
What Exactly Is a Chiral Center? Let's Break It Down.
Alright, let's get down to business. What makes an atom a "chiral center"? It's actually quite straightforward once you get the hang of it. Imagine a carbon atom. Carbon is pretty versatile, forming bonds with all sorts of other atoms. For a carbon atom to be a chiral center, it needs to be attached to four different groups. Yep, that's the magic number. Four distinct things. No two alike!
Think of it like this: if you have a carbon atom and it's bonded to, say, two hydrogen atoms and two other different groups, it's not chiral. Because it has two identical hydrogen atoms, it can be flipped over and look exactly the same. But if you swap one of those hydrogens for, let's say, a chlorine atom, and then you have four completely different things attached – your carbon is now a bona fide chiral center. Boom! Mind blown, right?
This is why the arrangement of these groups around the carbon atom is so important. It's not just about what is attached, but how it's arranged in 3D space. This leads to those mirror-image molecules we talked about, called enantiomers. They're like left and right hands – identical in composition but different in orientation.
Let's Get Our Hands Dirty (Metaphorically Speaking!) - Finding Chiral Centers
Now for the fun part! We're going to tackle some examples. Don't be intimidated by the chemical structures. We'll go through them step-by-step. Remember, we're looking for carbon atoms bonded to four different groups. Easy peasy, lemon squeezy.
Compound 1: 2-Chlorobutane
Okay, first up, let's look at 2-chlorobutane. The name itself gives us clues. "Butane" means four carbon atoms in a row. "2-chloro" means a chlorine atom is attached to the second carbon. Let's visualize it:
C - C - C - C
Now, let's add the atoms and the chlorine:
H Cl H H
| | | |
H-C - C - C - C-H
| | | |
H H H H
Now, let's examine each carbon atom, starting from the left (let's call them C1, C2, C3, and C4).
C1: This carbon is bonded to three hydrogen atoms and the rest of the molecule (C2). Since it has three identical hydrogens, it's not a chiral center. Nope, not this one.
C2: Ah, this one looks promising! This carbon is bonded to: 1. A hydrogen atom (H) 2. A chlorine atom (Cl) 3. The methyl group on its left (CH3, which is C1 and its attached hydrogens) 4. The ethyl group on its right (CH2-CH3, which is C3 and C4 and their attached hydrogens)
Are these four groups different? Yes! We have H, Cl, CH3, and CH2CH3. They are all distinct. Therefore, C2 is a chiral center. This is where the "handedness" of this molecule comes from!
C3: This carbon is bonded to two hydrogen atoms, C2, and C4. Since it has two identical hydrogen atoms, it's not a chiral center. Moving on!
C4: This carbon is bonded to three hydrogen atoms and C3. Three identical hydrogens means it's not a chiral center. Sad trombone.
So, in 2-chlorobutane, only one chiral center exists: the carbon at position 2.
Compound 2: Alanine (An Amino Acid)
Amino acids are the building blocks of proteins, and many of them are chiral! Let's look at alanine. It's one of the simpler ones. The general structure of an amino acid has a central carbon atom bonded to: 1. A hydrogen atom (-H) 2. An amino group (-NH2) 3. A carboxyl group (-COOH) 4. A side chain (which varies for each amino acid)
For alanine, the side chain is just a methyl group (-CH3).
So, let's draw it out, focusing on that central carbon, which we'll call the alpha-carbon (α-carbon). The formula is CH3-CH(NH2)-COOH.
NH2
|
H3C - C* - COOH
|
H
Let's examine the central carbon (marked with the asterisk ).
This carbon is bonded to:
- A hydrogen atom (-H)
- An amino group (-NH2)
- A carboxyl group (-COOH)
- A methyl group (-CH3)
Are these four groups different? Absolutely! We have H, NH2, COOH, and CH3. All completely unique. Therefore, the alpha-carbon in alanine is a chiral center. This is why there are two forms of alanine, L-alanine and D-alanine, which your body uses differently!
Compound 3: Glyceraldehyde
Glyceraldehyde is a simple sugar, and it's another classic example of a chiral molecule. Its structure is CH2OH-CH(OH)-CHO.
CHO
|
H2C-OH - C - OH
|
H
Let's focus on the central carbon (marked with ).
This carbon is bonded to:
- A hydrogen atom (-H)
- A hydroxyl group (-OH)
- A hydroxymethyl group (-CH2OH)
- An aldehyde group (-CHO)
Are these four groups different? You bet they are! H, OH, CH2OH, and CHO are all distinct. So, the central carbon in glyceraldehyde is a chiral center. It's one of the simplest chiral molecules you'll encounter!
Compound 4: Cholesterol (A Piece of it, Anyway!)
Okay, now things get a bit more complex. Cholesterol is a big molecule with lots of carbons. We're not going to analyze the whole thing (that would take ages!), but let's pick out a couple of potentially chiral carbons to illustrate. Remember, four different groups is our mantra!
Let's look at a section of the cholesterol molecule. We'll focus on carbons that are part of rings or have multiple different things attached. For brevity, I'll represent some parts of the molecule as R groups, but imagine them as specific arrangements of atoms.
Consider a carbon atom within one of the fused rings that also has a methyl group attached and another substituent coming off the ring. For example, a carbon that's part of a ring junction, bonded to two other carbons in the ring, a methyl group, and a hydrogen atom.
Let's imagine a carbon atom (let's call it C) within a ring:
CH3
|
Ring-C-- Ring-B
|
H
In this simplified view, C is bonded to:
- A methyl group (-CH3)
- A hydrogen atom (-H)
- One part of the ring (let's call it Ring-A)
- Another part of the ring (let's call it Ring-B)
Now, here's the tricky part. For C* to be chiral, the "Ring-A" path and the "Ring-B" path need to be different. In complex ring systems like cholesterol, they almost always are! The carbon atoms in the rings have different neighbors as you move around the ring, making the paths distinct. So, if we look at the actual structure of cholesterol, you'll find many carbons that fit this description: they are part of ring systems, bonded to a hydrogen, a methyl group, and two different paths along the rings. These are all chiral centers.
For instance, the carbon that has the hydroxyl group (-OH) attached to it in cholesterol is also chiral. It's bonded to the -OH group, a hydrogen, and two different parts of the ring system. So, that's another chiral center!
Identifying chiral centers in large, complex molecules like cholesterol is where practice really comes in. You have to meticulously trace the bonds and ensure that each of the four attachments to a carbon atom is truly unique. It's like navigating a maze – one wrong turn and you're off!
Compound 5: Isopropyl Alcohol (Propan-2-ol)
Let's try a super simple alcohol. Isopropyl alcohol, also known as propan-2-ol. The structure is CH3-CH(OH)-CH3.
OH
|
H3C - C - CH3
|
H
Let's examine the central carbon atom.
This carbon is bonded to:
- A hydroxyl group (-OH)
- A hydrogen atom (-H)
- A methyl group (-CH3)
- Another methyl group (-CH3)
Uh oh. Do we have four different groups here? Nope. We have two identical methyl groups. Because of this, this carbon atom is not a chiral center. This molecule is achiral – it's the same as its mirror image. No "handedness" here!
Why Does All This "Handedness" Stuff Matter?
So, you might be thinking, "Okay, so some molecules are like my hands. Big deal." Well, it is a big deal! Especially in biology and medicine. Remember our lopsided cake? Sometimes, the "left-handed" version of a molecule might fit perfectly into a receptor in your body, like a key in a lock, and do something wonderful. The "right-handed" version might not fit at all, or worse, it might fit in the wrong place and cause harmful side effects. Thalidomide is a famous, tragic example where one enantiomer was a sedative, and the other caused severe birth defects. Scary stuff!
Even in things we eat and drink, chirality plays a role. The difference between the smell of lemons and oranges? Chirality! The sweetness of one sugar versus the bitterness of another? Often due to chirality!
Understanding chiral centers helps chemists predict the properties of molecules and design new drugs, materials, and even flavors. It's a fundamental concept that underpins so much of what we understand about the molecular world.
Your Turn to Be a Chiral Detective!
I hope this little tour has made identifying chiral centers less daunting and more like a fun puzzle. The key is always to look for that carbon atom bonded to four different groups. Don't get distracted by carbons with double or triple bonds (they can't have four different groups) or carbons bonded to two or more identical atoms (like hydrogens or methyl groups in isopropyl alcohol).
Keep practicing with different molecules. Look up some common organic compounds and try to identify their chiral centers. You'll start to see patterns, and it will become second nature. It's a skill that will serve you well in chemistry, biochemistry, and beyond. Happy hunting, chiral detectives!
