Hydrogen Peroxide Catalyzed By Manganese Dioxide Balanced Equation
Ever feel like your science experiments are a bit… sleepy? Like they’re just humming along without any real pizzazz? Well, get ready to wake them up, because we’re diving into a reaction that’s got some serious sparkle! We’re talking about hydrogen peroxide doing its thing, but with a little help from a surprisingly energetic friend: manganese dioxide. It’s like a superhero duo, and their teamwork creates something pretty darn cool.
Think of hydrogen peroxide (that stuff you might use to clean a cut, but way more concentrated in a lab) as a molecule that’s just itching to break down. It’s kind of like a fizzy drink that’s been shaken up – it’s ready to release all that energy. But on its own, it can take its sweet time. It’s a bit of a slowpoke, really. It’ll eventually break into water and oxygen, but it’s not exactly a thrill ride.
Now, enter our trusty sidekick, manganese dioxide. This isn't some fancy, complicated chemical. It’s actually a dark brown powder that you can find in labs. It’s got this amazing ability to speed up reactions without actually getting used up itself. It's like the ultimate cheerleader! It just jumps in, gives the hydrogen peroxide a little nudge, and BAM! Things start happening much, much faster. It’s a catalyst, and it’s the star of our show in making this reaction so exciting.
So, what’s the big deal? Why is this little partnership so entertaining? It’s all about the speed and the drama! When manganese dioxide gets involved, that slow, sleepy breakdown of hydrogen peroxide turns into a lively, bubbly performance. You get a whole lot of oxygen gas produced, and it happens in a flash! It’s like watching a lazy river suddenly transform into a whitewater rapid – totally exhilarating.
Let's get to the nitty-gritty, the behind-the-scenes magic, which is the balanced equation. Don’t let that fancy term scare you! Think of it as the recipe for our chemical party. It tells us exactly how much of each ingredient we need and what we get out at the end. It’s super important in science to make sure we’re not creating or destroying any atoms – everything has to balance out perfectly. It's like baking a cake; you need the right amount of flour, sugar, and eggs to get a delicious result. Mess up the recipe, and you might end up with a brick!
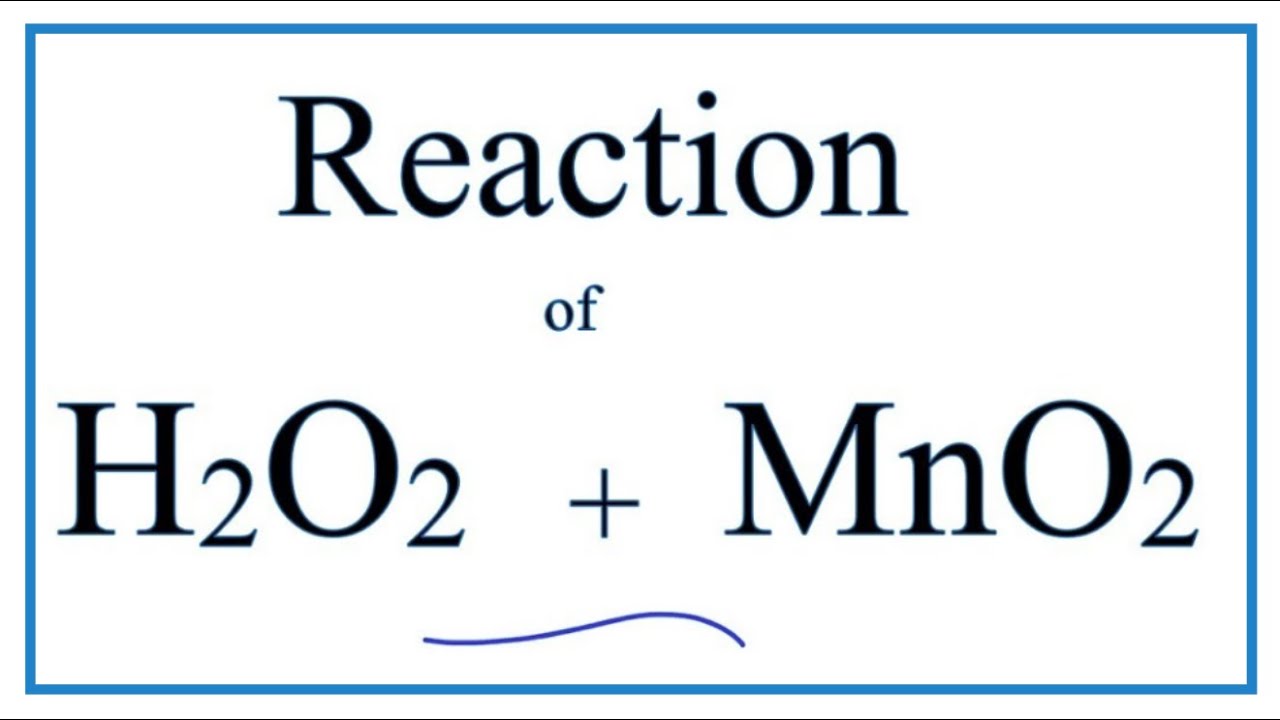
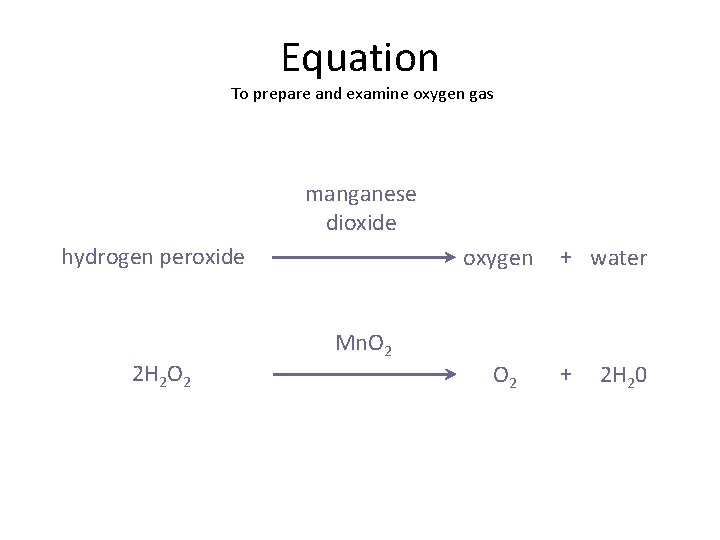
For our hydrogen peroxide and manganese dioxide extravaganza, the balanced equation looks like this:
2 H₂O₂ (aq) → 2 H₂O (l) + O₂ (g)
Whoa, what does all that mean? Let’s break it down, super simple.
First, you see 2 H₂O₂ (aq). The 'H₂O₂' is our hydrogen peroxide. The 'aq' just means it's dissolved in water, which is how it usually is when we use it. The '2' in front means we're starting with two molecules of hydrogen peroxide. Think of these as our two main performers, ready to put on a show.
Then you see the arrow (→). This is the "goes to" or "transforms into" part of our recipe. It’s the moment of change, the magic happening!
After the arrow, we have 2 H₂O (l). This is water! Yep, our energetic hydrogen peroxide breaks down into good old water. The 'l' just means it's in liquid form. Again, the '2' in front tells us we make two molecules of water.
And then there’s the really exciting part: O₂ (g). This is oxygen gas! The 'g' means it’s a gas. This is the stuff that makes things bubble and fizz, the visible sign of our reaction in full swing. And because it’s just 'O₂' and not '2 O₂', it means we get one molecule of oxygen gas produced from those two molecules of hydrogen peroxide.

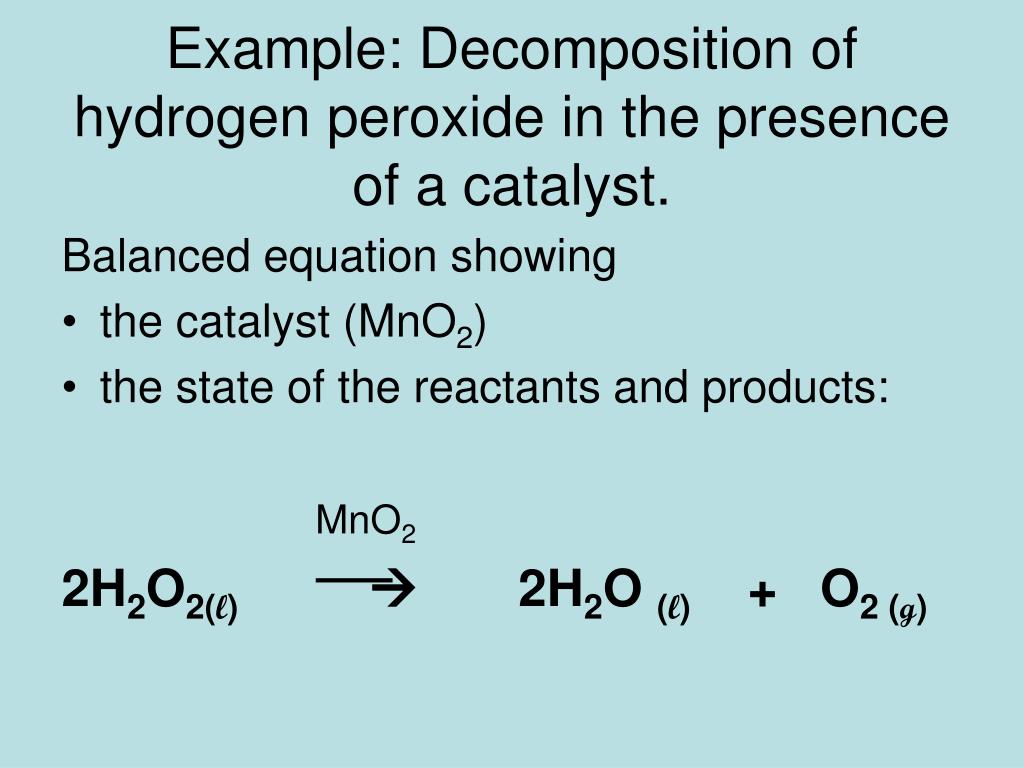
Now, where does our amazing manganese dioxide fit in? You might notice it’s not written in the main part of the equation like H₂O₂. That's because, as a catalyst, it's not a reactant or a product. It’s like the conductor of an orchestra. The conductor guides the music and makes it sound amazing, but they don’t become part of the music themselves. They’re just there to make everything work better and faster. In this reaction, manganese dioxide is written above the arrow, showing it’s facilitating the reaction but doesn't get consumed.
So, the full picture, with our star catalyst, looks something like this:
2 H₂O₂ (aq) ---MnO₂→ 2 H₂O (l) + O₂ (g)
See? The MnO₂ is perched right above the arrow, smiling down and making all the magic happen. It’s a tiny addition that makes a GIANT difference in how exciting this reaction is.
What makes this so entertaining to watch? It’s the sheer enthusiasm of the breakdown! When you add manganese dioxide to hydrogen peroxide, you don't just get a gentle fizz. You get a vigorous foaming, a rapid release of gas that can be quite dramatic. It’s visual, it’s energetic, and it’s a fantastic demonstration of how a little help can turn a slow process into a speedy spectacle.
It’s the kind of reaction that makes you go, "Wow!" You can literally see the oxygen gas being produced, creating foam and bubbles. It’s a tangible result of chemical change, and it’s pretty darn satisfying to observe. It’s not a reaction that requires complex equipment or super-dangerous conditions. It’s accessible, it’s observable, and it’s a great starting point for anyone curious about how the world of chemistry works.
This simple reaction, with its clear balanced equation and its explosive (in a good way!) output, is a brilliant example of catalysis. It shows us that sometimes, all it takes is the right partner to unlock incredible potential. It’s a reminder that even common substances can have hidden talents and that chemistry can be, dare I say, fun and engaging. So next time you hear about hydrogen peroxide and manganese dioxide, remember the dynamic duo and the exciting chemical dance they perform!
