How Many Water Molecules Could Hydrogen Bond Directly To Glucose

Imagine you have a tiny, sweet molecule called glucose. It's the sugar that gives us energy! Now, think about water molecules. They're like little magnets, always wanting to be close to other things.
So, how many of these water magnets can hug our glucose molecule at once? It's a fun little puzzle! It's not just one or two; it's a whole bunch.
This is where the magic of hydrogen bonding comes in. It’s like a special handshake between water and glucose. These handshakes are super important for how glucose acts in our bodies.
Glucose has these little sticky spots, called hydroxyl groups. Think of them as tiny hands reaching out. Water molecules have their own little sticky spots too.
When a water molecule gets close, its sticky bits can grab onto glucose's sticky bits. It's a delightful dance of attraction. This is what we call a hydrogen bond.
Now, the fun part is figuring out how many of these bonds can form. It's like a party, and everyone wants a dance partner! Glucose isn't a shy molecule.
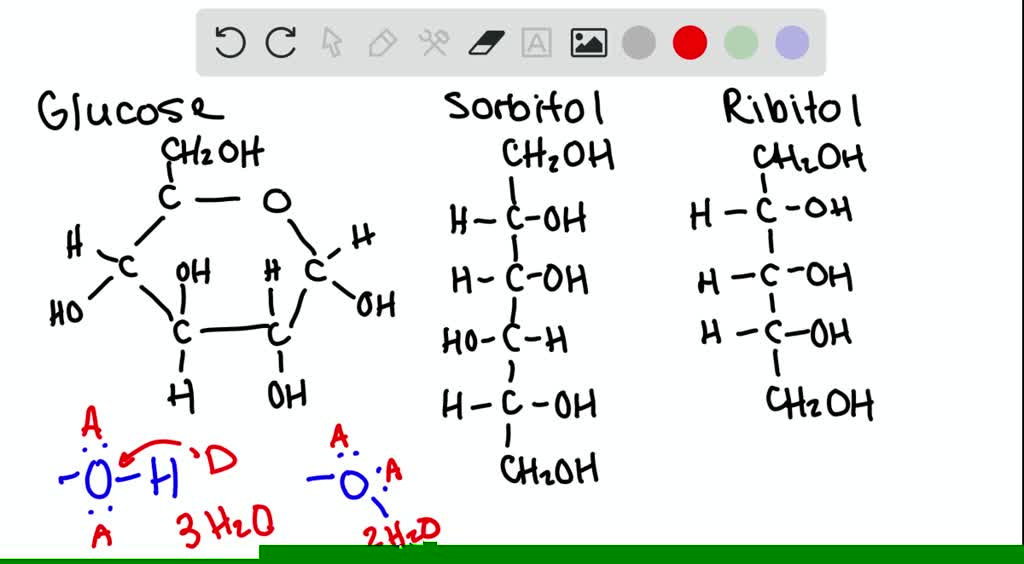
It has not just one or two, but five of those hydroxyl groups. That means it has five potential places to grab a water molecule. Wow!
So, in theory, glucose could form five direct hydrogen bonds. That’s a lot of little water buddies hanging out! Each bond is a tiny, temporary connection.
But here's where it gets even more interesting. It's not always a perfect one-to-one situation. Sometimes, the water molecules are busy holding hands with each other.
Water is famous for forming its own network of hydrogen bonds. It's like a big, happy family reunion where everyone is already holding hands. This can affect how many water molecules can reach glucose.
So, while glucose can make five direct bonds, the actual number might be a bit fluid. It depends on the surrounding environment. Is there a lot of free water around?
Think of it like this: you have a toy with five hooks, and you have a pile of toy rings. You could hang five rings. But if the rings are already linked together, you might only get one or two onto your toy at a time.
This is why studying these interactions is so fascinating. It’s not just a simple count. It’s about the dynamic interplay between molecules.
These hydrogen bonds are the unsung heroes of biology. They keep things dissolved, they help build structures, and they power chemical reactions. Pretty neat, huh?

For glucose, these bonds are crucial for its journey through our bloodstream. Water is the medium, and hydrogen bonds are the transportation system.
Imagine glucose trying to travel. It needs to be soluble, meaning it needs to mix well with water. Hydrogen bonds make this happen. They make glucose feel at home in water.
Without these bonds, glucose would be like a lost tourist, unable to navigate the watery world of our cells. It wouldn't get to where it needs to go.
So, back to our original question: how many water molecules? It's at least five potential sites. But in reality, it's a dynamic number. It fluctuates!
Scientists use fancy tools and clever experiments to figure this out. They're like molecular detectives, piecing together clues. It's a constant exploration.
The beauty of it is that even though we can't see these bonds with our naked eye, they are incredibly powerful. They shape the world around us.
Think about how water can climb up a plant stem. That’s partly thanks to hydrogen bonding. It’s a small force with big consequences.
For glucose, the number of water molecules directly attached isn't fixed. It's more like a range. It’s a lively, active scene.
When glucose is floating around in your body, it's surrounded by a crowd of water molecules. Some are directly holding hands, others are just nearby, part of the general water crowd.
The five hydroxyl groups are the stars of the show, the primary handshake locations. But the surrounding water molecules also play a role. They influence how well glucose can interact.
It’s like a dance floor. Glucose is the dancer, and water molecules are the partners. Sometimes, the dancer has five hands free to hold. Other times, the dancer's hands are busy holding other dancers’ hands.
This dance of water and glucose is happening all the time inside you. It’s part of the amazing symphony of life. Isn’t that cool?
The exact number can vary depending on things like temperature and the concentration of glucose and water. It’s a constantly changing picture.
But the potential is key. Glucose is set up to make many hydrogen bonds. It’s designed to interact with water.
So, while a precise, single number might be elusive, the idea of glucose being able to form many direct hydrogen bonds with water is what’s truly important. It's the foundation of its function.
It’s a reminder that even the smallest things in nature are incredibly complex and interconnected. They’re like tiny, intricate machines.
If you ever get curious about the tiny world inside you, this is a great place to start. It’s a world of invisible forces and amazing partnerships.
So, the next time you enjoy something sweet, remember the glucose molecule. It’s a busy little guy, forming bonds and fueling your day.
And remember the water molecules, always ready to lend a hand, or in this case, a hydrogen bond. They’re the best of friends.
The number might be fluid, but the principle is clear: glucose loves water, and water loves glucose. It’s a match made in molecular heaven.
It’s a beautiful example of how chemistry makes life possible. It’s happening right now, in every living thing.
So, while we can say glucose has five hydroxyl groups ready to bond, the actual number of water molecules directly interacting is a dynamic dance. It’s a number that's always changing, always fascinating.
It’s a constant invitation to explore the wondrous world of molecules and their interactions. Who knew sugar and water could be so exciting?
The exploration of these molecular interactions is a journey into the very essence of life. It's a testament to the elegant simplicity and profound complexity of the natural world.
It’s a simple question with a complex and beautiful answer. The more you learn, the more you realize how much there is to discover.

So, the next time you see a drop of water, or think about food, remember the tiny molecules at work. They’re doing amazing things!
It’s a reminder that even the smallest interactions can have huge impacts. They are the building blocks of everything.
This simple act of hydrogen bonding is what allows glucose to be transported and utilized by our bodies. It’s a fundamental process.
The number of water molecules that can form a direct hydrogen bond with glucose isn't a static figure. It’s more of a potential and a dynamic equilibrium.
The five hydroxyl groups are the key players, but the surrounding water molecules also influence the overall picture. It’s a collaborative effort.
Think of it as a party. Glucose is the host with five arms ready to greet guests. But sometimes, guests are already dancing with each other, so the host might only get to hold a few hands at a time.
This is the charm of molecular science. It’s about understanding these intricate dances and how they shape our world.
It’s a constant source of wonder, a glimpse into the hidden machinery that keeps life running smoothly.
The world of molecules is vast and full of surprises. And the interaction between glucose and water is just one tiny, sweet example.
So, dive in and explore! There’s always something new and exciting to discover in the world of chemistry.
The beauty lies in the simplicity of the concept and the profound implications it holds for biological processes.
It's a delightful dance of attraction, a testament to the power of intermolecular forces.

So, while a definitive number is hard to pin down, the capacity for multiple hydrogen bonds is what makes glucose so vital.
It’s a testament to nature’s ingenious design, where even a simple sugar molecule plays a critical role.
The exploration of these molecular dynamics is a journey that continues to reveal new insights and inspire awe.
It's a reminder that even the smallest units of matter are engaged in constant, fascinating interactions.
The more we understand, the more we appreciate the intricate web of life.
This dance of hydrogen bonds is fundamental to how our bodies function, from energy production to cellular communication.
It’s a truly captivating aspect of chemistry that impacts our lives in countless ways.
So, the next time you eat something sweet, give a little nod to the amazing world of molecular interactions!
The question might seem simple, but the answer is a window into a much larger, intricate system.
It’s a beautiful illustration of how chemistry underpins all biological processes.
And the story of glucose and water is just the beginning of a fascinating molecular adventure.
It’s a constant interplay of forces that keeps the world alive and thriving.
