How Many Different Kinds Of Protons Are Present In 1-chlorohexane
Hey there, fellow curious minds! Ever find yourself staring at a perfectly brewed cup of coffee, or perhaps admiring the sleek lines of your smartphone, and suddenly get hit with a burning question about the tiny, invisible building blocks that make it all happen? Yeah, me too. Today, we're diving into a world that might sound a little bit science-y, but trust me, it’s more like uncovering a hidden, fascinating puzzle. We’re talking about protons, specifically in a molecule called 1-chlorohexane. Sounds intimidating? Not at all! Think of it as getting to know the “personalities” of the protons in this particular molecule. It’s all about understanding how they’re different, and why that difference actually matters.
So, what exactly are we even talking about? Protons, in the grand scheme of things, are fundamental particles found in the nucleus of every atom. They're like the tiny, positively charged heart of an atom. But here's where it gets interesting: in organic chemistry – the chemistry of carbon-based life and materials – we often focus on how atoms are arranged and how they interact. And it’s the environment around a proton that makes it unique. Imagine a celebrity at a party. They're still a person with a heart, but the way they interact with the paparazzi, their entourage, or a quiet corner booth at the bar? That's their unique "proton environment."
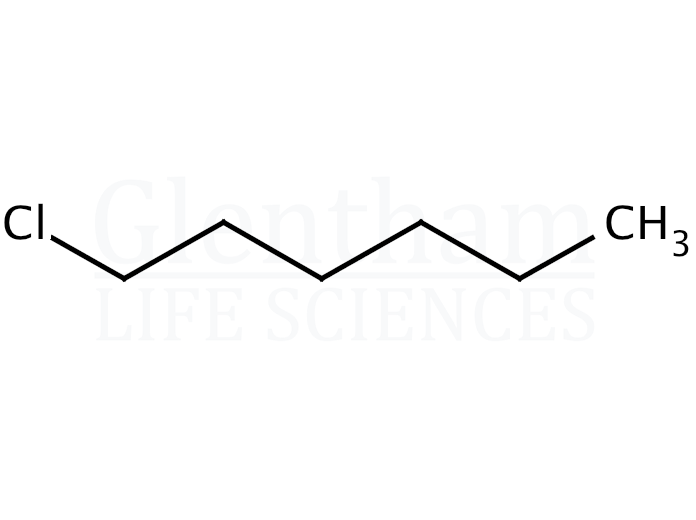
Let’s introduce our star player: 1-chlorohexane. What is this thing? Picture a chain of six carbon atoms all linked together, like little links in a bracelet. Now, on one end of this chain (the "1" position, you see), we've attached a chlorine atom. Chlorine is a bit of a diva – it’s quite electronegative, meaning it loves to hog electrons. This makes our 1-chlorohexane molecule a bit polarized, like a magnet with a strong North and South pole. It's a common organic molecule, used in various industrial processes and sometimes even found as an intermediate in the synthesis of other compounds. Think of it as a basic building block, like a LEGO brick that can be used to construct more complex things.
Now, the question is: how many different kinds of protons are there in 1-chlorohexane? This isn’t about the total number of protons, but rather how many distinct "environments" these protons experience. Protons are considered different if they are in non-equivalent positions within the molecule. We can determine this by looking at the molecular structure and considering symmetry. If you can’t swap one proton for another through a symmetry operation (like a rotation or reflection) and have the molecule look exactly the same, then they are likely different kinds of protons. It’s like having identical twins; they might look the same, but one might prefer coffee while the other is a tea enthusiast. Their experiences, their "environments," are different.
Unraveling the Proton Parade
Let’s break down our 1-chlorohexane molecule, C6H13Cl, and count these unique proton personalities. Remember, we’re looking for distinct chemical environments. This is where the magic of Nuclear Magnetic Resonance (NMR) spectroscopy comes into play. While we won't be doing any actual spectroscopy here, the principles are what help us figure this out. NMR is like giving each proton a unique fingerprint based on its surroundings. Different fingerprints mean different kinds of protons!
Our 1-chlorohexane molecule has the following structure: Cl - CH2 - CH2 - CH2 - CH2 - CH2 - CH3.
Let’s go from the end closest to the chlorine and work our way down the chain. We’ll label the carbon atoms C1, C2, C3, C4, C5, and C6, starting with the carbon attached to chlorine as C1.
The Chlorine Crew (C1 and its protons)
First up, we have the CH2 group directly attached to the chlorine atom. This is C1. The two hydrogens on this carbon are bonded to it. Because of the strongly electronegative chlorine atom, these two hydrogens are in a very specific environment. The chlorine atom pulls electron density away from the carbon, and this effect is felt by the hydrogens attached to it. These two hydrogens are chemically equivalent due to the free rotation around the C1-C2 bond. Think of them as being in the "influencer zone." So, we have one distinct type of proton here.
Fun Fact: The C-Cl bond is polar, making this part of the molecule a bit like the intro of a catchy song – it sets the tone for the rest of the molecule!
The Next Door Neighbors (C2 and its protons)
Moving along to C2, we have another CH2 group. This carbon is bonded to C1 and C3. The protons on C2 are influenced by the chlorine (though to a lesser extent than those on C1) and by the rest of the carbon chain. They are in a different electronic environment than the protons on C1. Imagine the difference between being front-row center at a concert versus being in the VIP section. Both are great, but the experience is distinct. So, these two hydrogens on C2 represent a second distinct type of proton.
Cultural Nugget: This concept of "neighboring effects" is something we see everywhere, from how different ingredients in a recipe blend together to how different genres of music can influence each other.
The Middle of the Pack (C3 and C4, and their protons)
Now, let's look at C3 and C4. These are both CH2 groups, and they are located in the middle of the hexane chain. Crucially, they are chemically equivalent to each other due to the symmetry of the hexane chain. Imagine a perfectly balanced scale; C3 and C4 are like the weights on either side. The electron-withdrawing effect of the chlorine is quite diminished by the time it reaches these carbons. Therefore, the two protons on C3 are in the same chemical environment as the two protons on C4. They are experiencing similar electronic influences from the rest of the chain. This gives us a third distinct type of proton.
Practical Tip: When you’re cooking, think about how ingredients in the middle of a dish might develop similar flavors from the surrounding components, while those at the edges might have a more distinct profile. It’s all about the environment!
Nearing the End (C5 and its protons)
We're getting closer to the other end of the chain! C5 is another CH2 group. Its environment is distinct from C1, C2, C3, and C4. It's influenced by the CH3 group next to it, and it’s further away from the chlorine than C1, C2, C3, and C4. This gives us a fourth distinct type of proton.
Fun Fact: As you move further down a chain of atoms, the influence of a substituent at one end tends to decrease. It's like a ripple effect in water – the further from the stone drop, the weaker the ripple.
The Party Animal at the End (C6 and its protons)
Finally, we reach C6, which is a CH3 group. These three protons are all equivalent to each other due to the free rotation around the C5-C6 bond. They are at the very end of the chain, far from the chlorine atom. Their environment is unique and different from all the other protons we've discussed. This represents our fifth distinct type of proton.
Cultural Reference: Think of the methyl group (CH3) as the energetic friend at the end of the party, perhaps singing karaoke or telling jokes, while the protons closer to the chlorine might be the more contemplative ones discussing the meaning of life.
Putting It All Together: The Proton Count
So, let's recap our distinct proton environments in 1-chlorohexane:
- The two protons on C1 (directly attached to chlorine): Type 1
- The two protons on C2: Type 2
- The four protons on C3 and C4 (these are chemically equivalent): Type 3
- The two protons on C5: Type 4
- The three protons on C6 (the methyl group): Type 5
Therefore, in 1-chlorohexane, there are five different kinds of protons! Each type of proton experiences a unique electronic environment, which influences its chemical behavior and how it interacts with other molecules. This is precisely what makes NMR spectroscopy so powerful for chemists – it allows them to distinguish between these different proton types and deduce the structure of unknown molecules.
Visual Aid Idea: Imagine a timeline of the molecule. Protons near the chlorine are like the early birds, heavily influenced by it. Protons in the middle are like the steady middle-class, with a more balanced influence. Protons at the far end are the independent spirits, with minimal influence from the chlorine.
Why Does This Even Matter in the Real World?
You might be thinking, "Okay, five kinds of protons. Cool story, but how does this relate to my everyday life?" Great question! Understanding the different types of protons and their environments is fundamental to chemistry, and chemistry is everywhere. It's in the food we eat, the clothes we wear, the medicines that heal us, and the technologies that power our world.
For instance, when scientists design new drugs, they need to understand how molecules will interact with our bodies. The subtle differences in the electronic environment of protons (and other atoms) can dictate whether a drug binds to a specific target protein or not. It’s like having a lock and key; the shape and electronic "feel" of the key (the drug molecule) have to match the lock (the biological target).
In material science, understanding molecular structures and the properties they impart is key. Whether it's creating stronger plastics, more efficient solar cells, or even developing better non-stick pans, it all comes down to manipulating molecules at a fundamental level. The differences in proton environments contribute to the overall physical and chemical properties of a substance – its reactivity, its solubility, its melting point, and so on.
Even in something as seemingly simple as baking, the principles are at play. The way ingredients interact and change during baking is a complex series of chemical reactions. Understanding the structure of molecules like sugars and proteins, and how their protons are positioned, helps us predict how they'll behave under heat, leading to perfectly baked cakes or crispy cookies.
Everyday Analogy: Think about your social circle. You have different types of friends, right? The energetic one, the quiet observer, the wise advisor, the silly comedian. Each friend brings a different dynamic to your life. Similarly, each type of proton brings a unique characteristic to the 1-chlorohexane molecule.
A Little Reflection
It’s quite mind-blowing when you stop to think about it, isn’t it? We’re surrounded by molecules, each with its own intricate design and a multitude of tiny components like protons, all playing their part. The fact that even within a single, relatively simple molecule like 1-chlorohexane, there are distinct "personalities" of protons, each subtly influenced by its surroundings, is a testament to the complexity and elegance of the universe. It reminds us that often, the most profound insights come from looking closely at the seemingly small details. So next time you’re sipping that coffee or scrolling through your feed, take a moment to appreciate the invisible world of molecules and the fascinating diversity of their tiny, fundamental building blocks. It’s all part of the grand, ongoing story of matter.
