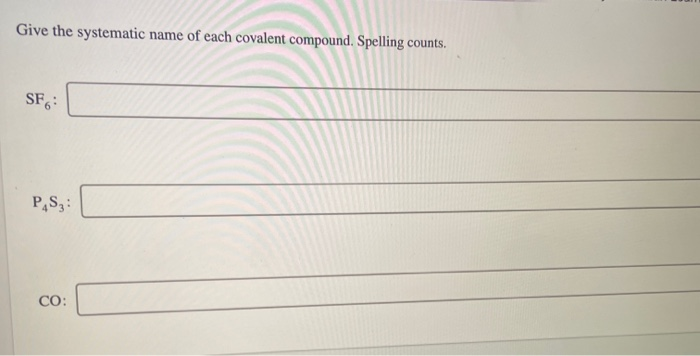
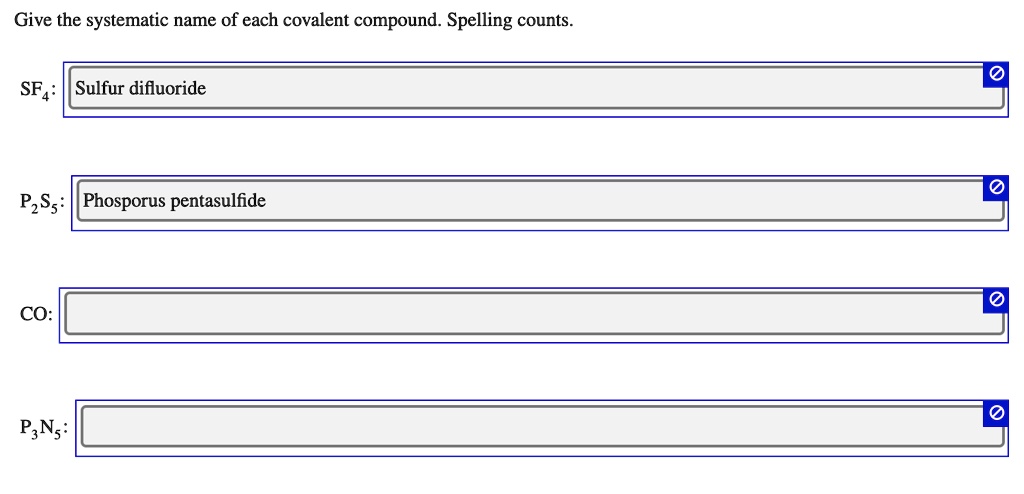
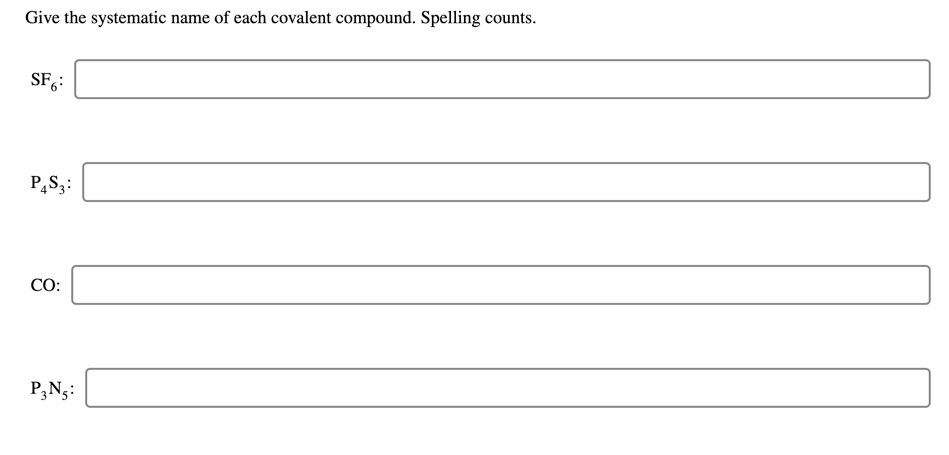
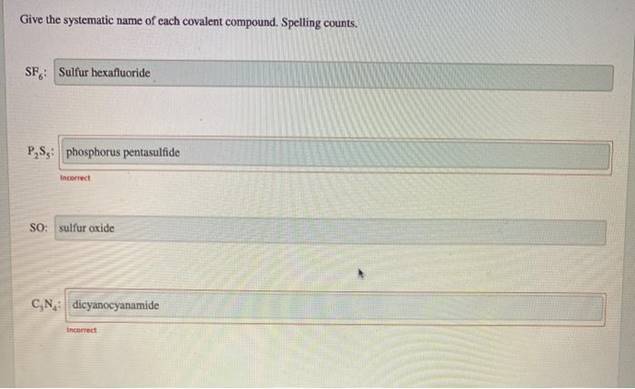
Give The Systematic Name Of Each Covalent Compound. Spelling Counts.
I remember back in middle school, during one particularly bewildering chemistry class, our teacher, Mrs. Henderson, a woman whose hair seemed to defy gravity in a way that rivaled some of her periodic table elements, held up two bottles. One was labeled "H₂O," and the other, well, it looked suspiciously similar but was just called "water." She paused, a mischievous glint in her eye, and said, "Class, one of these is the scientific shorthand, the other is what you use to quench your thirst. Today, we’re learning how to give these chemical buddies their proper, systematic names. And trust me, it’s less about shouting into the void and more about carefully constructing a label."
Honestly, at the time, it felt like she was speaking a different language. Covalent compounds? Systematic names? My brain was still trying to grasp why an atom would even want to share electrons. But over time, and with a healthy dose of practice (and maybe a few late-night YouTube tutorials), I realized Mrs. Henderson was onto something. It’s like knowing someone’s full name versus just their nickname. While "water" is perfectly fine for asking for a drink, in the scientific world, clarity and precision are king. And when it comes to those sneaky, electron-sharing pals – the covalent compounds – their systematic names are the key to unlocking their identities.
So, why the fuss? Imagine trying to order a specific type of pizza with only vague descriptions. "Uh, I'd like one with, like, cheesy stuff and some tomato goo." Not very helpful, right? You need to know which cheese, what kind of goo, how many toppings, and so on. Chemical compounds are no different. They're built from atoms, and the way those atoms are arranged, and how many of each, dictates the compound's properties. The systematic name is our way of precisely describing this atomic arrangement.
Covalent compounds, for those of you who might have snoozed through that part of chemistry (no judgment here, I’ve been there!), are formed when atoms, usually nonmetals, share electrons to achieve a stable electron configuration. Think of it as a cooperative effort, unlike ionic compounds where one atom generously gives an electron to another. These sharing partnerships create molecules, and each unique molecule deserves its own unique, descriptive name.
The International Union of Pure and Applied Chemistry, or IUPAC for short (say it with me: "ee-yoo-pak"), is basically the global committee that sets the rules for naming chemical substances. They’re the ultimate arbiters of chemical nomenclature. And their system for naming covalent compounds is pretty darn logical once you get the hang of it. It’s like learning a secret handshake, but way more useful for, you know, understanding the universe.
The core of this naming system for binary covalent compounds (that's compounds made of just two different elements) relies on a set of numerical prefixes. These prefixes tell you how many atoms of a particular element are present in the molecule. It’s like having a little numerical decoder ring!
Let’s break down the prefixes. They’re pretty straightforward:
- 1 = mono- (but we usually drop this for the first element, unless it's absolutely necessary for clarity, which is rare.)
- 2 = di-
- 3 = tri-
- 4 = tetra-
- 5 = penta-
- 6 = hexa-
- 7 = hepta-
- 8 = octa-
- 9 = nona-
- 10 = deca-
See? Not too intimidating, right? You might have encountered some of these before in other contexts. "Tricycle" has three wheels, "decade" has ten years. It’s all connected!
Now, how do we apply these prefixes to name a compound? It’s a two-step process, really. First, you identify the elements involved. Then, you use the prefixes to indicate the number of each atom.
The rule is: you name the first element in the formula, followed by the second element, but with a crucial modification to the second element’s name. Its ending is changed to -ide.
Let's take our old friend, H₂O. We have Hydrogen (H) and Oxygen (O). According to the formula, there are two Hydrogen atoms and one Oxygen atom.
So, we start with the first element, Hydrogen. There are two of them, so we’d think "di-hydrogen." But wait! Remember that rule about dropping "mono-" for the first element? Well, it applies here too. If the first element only has one atom, we don't usually use "mono-." So, for H₂, it's just "hydrogen." (If the formula were, say, HO, we might see "monohydrogen," but that’s less common for simple binary compounds).
Now for the second element, Oxygen. There’s one Oxygen atom. So, we would use the prefix "mono-." But! And this is a big but, a very important but: if the prefix for the second element is "mono-," and it’s followed by a vowel (like the 'o' in oxide), we often drop the 'o' from the prefix to avoid a double vowel sound. So, "mono-oxide" becomes monoxide. And the ending of Oxygen changes to "-ide."
Putting it together: Hydrogen monoxide. Now, you might be thinking, "Wait a minute, Mrs. Henderson said 'water'!" And you’d be right! "Water" is the common name for H₂O, and it’s so widely used that it’s accepted even in scientific contexts. It’s like how everyone knows what a "car" is, even though its systematic name in a more technical classification might be more complex. But for learning purposes, the systematic name is key.
Let’s try another one. How about CO? We have Carbon (C) and Oxygen (O). One Carbon atom, one Oxygen atom.
First element: Carbon. There’s one, but we drop the "mono-" for the first element. So, carbon.
Second element: Oxygen. There’s one, so we use "mono-." And because it's followed by a vowel in "oxide," we drop the 'o' from mono-. So, monoxide. Changing the ending of Oxygen to "-ide."
Putting it together: Carbon monoxide. This is a very different compound from CO₂, which is carbon dioxide. Even a slight change in the number of atoms makes a big difference!
Okay, what about CO₂? Carbon and Oxygen again. One Carbon atom, two Oxygen atoms.
First element: Carbon. One atom, so just carbon.
Second element: Oxygen. Two atoms! So, we use the prefix di-. And the ending of Oxygen changes to "-ide."
Putting it together: Carbon dioxide. Ah, the stuff we exhale and that makes fizzy drinks bubble! See how crucial those prefixes are?
Let’s get a bit more complex. Consider SO₂. Sulfur (S) and Oxygen (O). One Sulfur atom, two Oxygen atoms.
First element: Sulfur. One atom, so just sulfur.
Second element: Oxygen. Two atoms, so di-. And the ending changes to "-ide."
Result: Sulfur dioxide.
Now, what about SO₃? Sulfur (S) and Oxygen (O). One Sulfur atom, three Oxygen atoms.
First element: Sulfur. One atom, so just sulfur.
Second element: Oxygen. Three atoms, so tri-. And the ending changes to "-ide."
Result: Sulfur trioxide. Notice the subtle difference in the name, reflecting the different molecular structure.
It’s important to remember that these rules primarily apply to binary covalent compounds – those made of two different nonmetal elements. For compounds involving metals and nonmetals, you’re usually dealing with ionic compounds, and they have their own set of naming rules (which are a whole other ballgame, but also fascinating!).
Let’s try some with prefixes other than di- and tri-.
How about PCl₃? Phosphorus (P) and Chlorine (Cl). One Phosphorus atom, three Chlorine atoms.
First element: Phosphorus. One atom, so phosphorus.
Second element: Chlorine. Three atoms, so tri-. And the ending changes to "-ide."
Result: Phosphorus trichloride.
And PCl₅? Phosphorus (P) and Chlorine (Cl). One Phosphorus atom, five Chlorine atoms.
First element: Phosphorus. One atom, so phosphorus.
Second element: Chlorine. Five atoms, so penta-. And the ending changes to "-ide."
Result: Phosphorus pentachloride.
You're getting the hang of it! It’s like assembling a chemical Lego set with very specific instructions.
Let’s test your understanding with something a little more… elaborate. What about N₂O? Nitrogen (N) and Oxygen (O). Two Nitrogen atoms, one Oxygen atom.
First element: Nitrogen. Two atoms, so di-. So, dinitrogen.

Second element: Oxygen. One atom, so mono-. And it’s followed by a vowel in "-ide," so we drop the 'o' from mono-. monoxide. Ending changes to "-ide."
Result: Dinitrogen monoxide. This one is also known as nitrous oxide, or laughing gas! Isn't it cool how the systematic name tells you exactly what's going on?
One more for good measure: N₂O₄. Nitrogen (N) and Oxygen (O). Two Nitrogen atoms, four Oxygen atoms.
First element: Nitrogen. Two atoms, so di-. Dinitrogen.
Second element: Oxygen. Four atoms, so tetra-. Ending changes to "-ide."
Result: Dinitrogen tetroxide.
Sometimes, when you have a prefix ending in 'a' or 'o' and the element name starts with a vowel, you might see some minor spelling adjustments for pronunciation. For example, with Sulfur and Oxygen, if you had something like S₂O₃ (disulfur trioxide), the 'o' in tri- is kept. But if you had something like S₂O₅ (disulfur pentaoxide), you might see the 'o' in penta dropped to avoid the double vowel, making it "pentoxide." IUPAC has specific rules for these, but the general idea is to make it sound less like you're gargling.
The key takeaway here is that the systematic name is a highly descriptive label. It removes all ambiguity. When a chemist sees "dinitrogen monoxide," they immediately know it's a molecule with two nitrogen atoms and one oxygen atom, arranged in a specific way that gives it its unique properties. It's far more precise than a common name, which might evolve over time or be regionally specific.
This system is incredibly powerful because it allows us to communicate about chemical substances with absolute clarity, no matter where in the world we are. It’s a universal language for chemists. And the more you practice, the more these names will roll off your tongue (or at least, the more easily you'll be able to decipher them).
So, next time you see a chemical formula like SF₆, don't panic. Break it down. What are the elements? How many of each atom are there? Apply the prefixes. Change the ending. You've got this! It’s Sulfur (S) and Fluorine (F). One Sulfur, six Fluorines. That makes it Sulfur hexafluoride. Pretty neat, huh?
Learning these systematic names might seem a bit like memorizing a dictionary at first, but it’s actually about understanding a logical structure. It’s about recognizing patterns. And once you see the pattern, the whole world of chemical nomenclature opens up. It’s a fundamental skill for anyone delving into chemistry, and it’s the foundation for understanding more complex chemical compounds and reactions. So, embrace the prefixes, master the -ide endings, and you'll be naming covalent compounds like a pro in no time. Happy naming!
