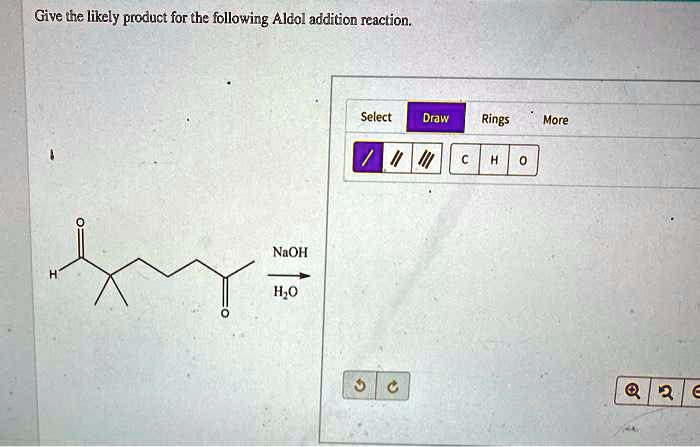
Give The Likely Product For The Following Aldol Addition Reaction.
Ever wondered what goes on behind the scenes when we whip up delicious baked goods or even when our bodies create essential molecules? Sometimes, it involves a bit of chemical magic, and one of the coolest tricks in the chemist's toolkit is called the Aldol Addition reaction. Think of it as a molecular handshake where two carbon-containing building blocks come together to form something bigger and more complex. It's not just for super-serious scientists; understanding this reaction can be surprisingly fun and give you a new appreciation for the chemistry all around us.
So, what's the big deal about the Aldol Addition? Its main purpose is to create new carbon-carbon bonds, which is fundamental to building larger organic molecules. This is like snapping LEGO bricks together to make something more intricate. The benefits are huge! This reaction is a cornerstone in organic synthesis, meaning it's a vital step in creating many of the medicines, plastics, flavors, and fragrances we use every day. Without it, the chemical world would be a much simpler, and less interesting, place.
You might be surprised to learn that the principles of Aldol Addition, or reactions very similar to it, are at play in various educational settings and even in daily life. In university chemistry labs, students meticulously learn to perform and predict these reactions, building their understanding of molecular construction. Beyond the classroom, the creation of many food flavorings, like the buttery aroma of diacetyl, can involve Aldol-like processes. Even certain biological pathways in our bodies use enzymes to facilitate similar bond-forming steps, essential for life itself.
Now, you might be thinking, "This sounds complicated!" But you can explore the concept in simple ways. A great starting point is to visualize the process. Imagine two molecules, one acting as a "donor" with a willing "active hydrogen" and the other as an "acceptor" with a carbonyl group (a carbon double-bonded to oxygen). When a catalyst (often a base or acid) is present, the donor molecule loses its active hydrogen and becomes reactive, ready to attack the acceptor. It’s like one molecule gets a little "activated" and then reaches out to grab onto another.
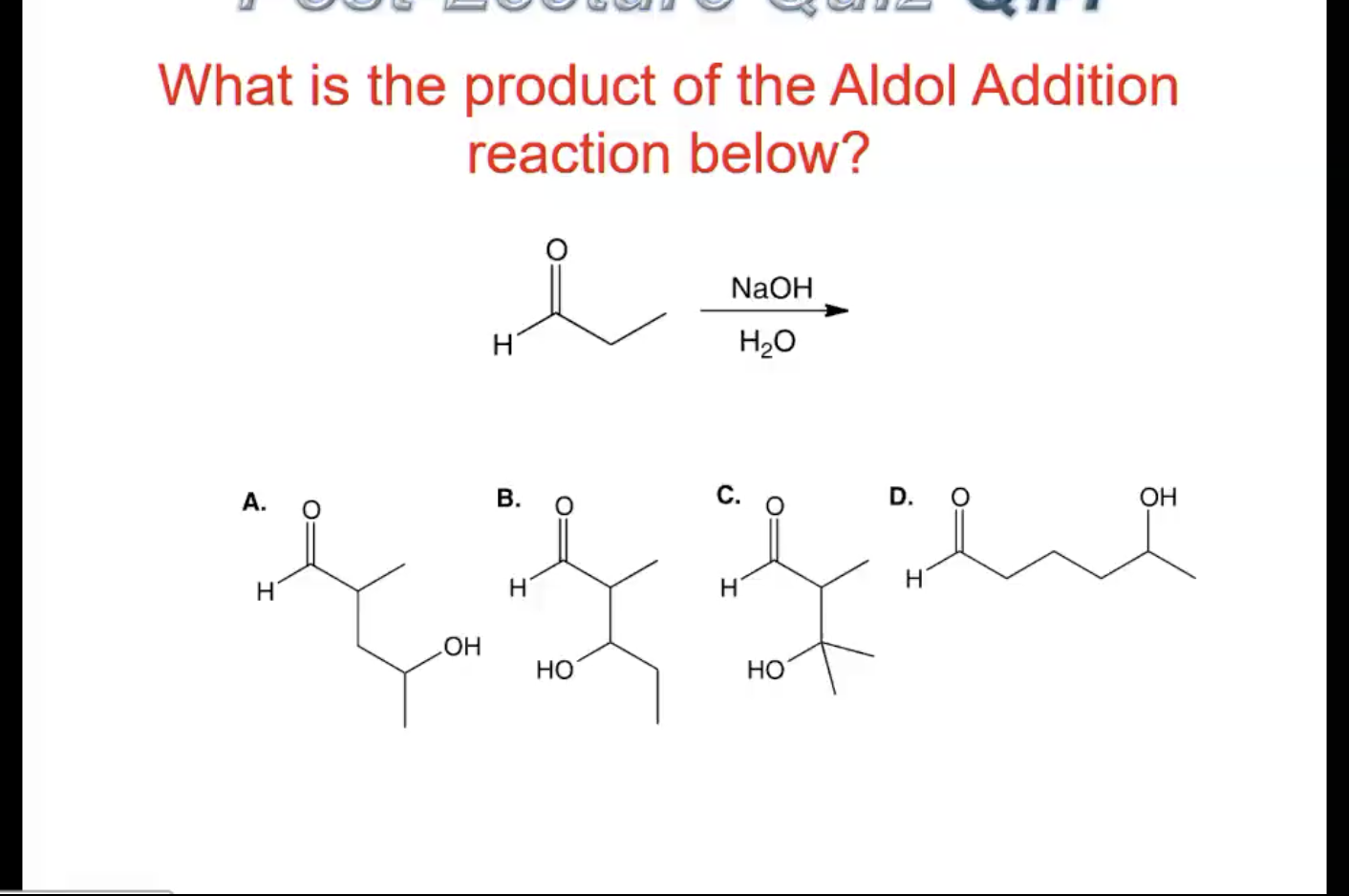
Let's consider a classic example. If we have acetaldehyde (which has a carbonyl group and an alpha-hydrogen) reacting with itself under basic conditions, the acetaldehyde acts as both the donor and acceptor. One molecule of acetaldehyde loses an alpha-hydrogen, forming an enolate ion. This enolate ion then attacks the carbonyl carbon of another acetaldehyde molecule. The result is a new, larger molecule with both an alcohol (-OH) group and a carbonyl group. This specific product is called 3-hydroxybutanal. It's a straightforward illustration of how two small molecules can join to form a bigger one, showcasing the core of the Aldol Addition.
To explore this further, you could look up diagrams and animations of the Aldol Addition online. Seeing the electrons move and bonds form can be incredibly helpful. Don't be afraid to search for "Aldol Addition mechanism" – visual aids make a world of difference. You can also try sketching out simple examples yourself. Pick two molecules with carbonyl groups and think about where the reaction might occur. It's a fantastic way to develop your chemical intuition and appreciate the elegant ways molecules can combine to create the world we know.
