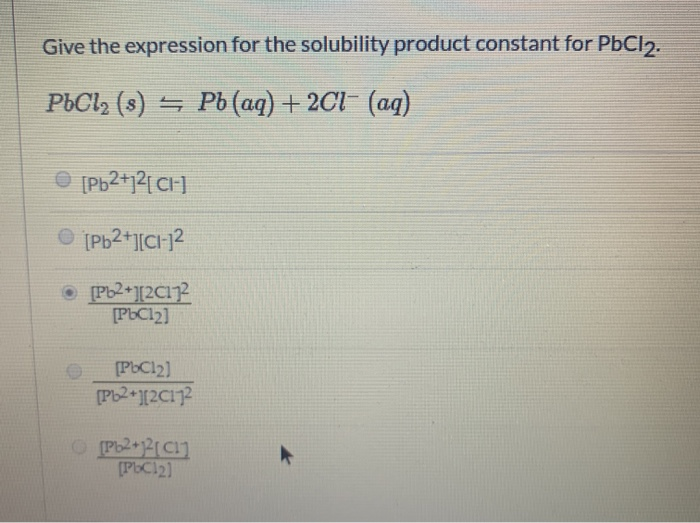
Give The Expression For The Solubility Product Constant For Pbcl2
Ever wondered what happens when you mix certain things together, especially in water? It's like a tiny, invisible party where some things break apart and others decide to stay whole. Today, we're peeking into the world of lead(II) chloride, or PbCl2.
Now, PbCl2 might sound a bit fancy, but it's actually a common compound. Think of it as a salt, a bit like the salt you put on your fries, but with a lead twist! And when you toss this particular salt into water, something quite interesting unfolds.
It doesn't just disappear completely like table salt does. Instead, a very tiny bit of it decides to dissolve. This is where the magic, or rather the science, starts to get really fun.
The Tiny Dance of Dissolving
Imagine PbCl2 as a bunch of little building blocks, each made of one lead(II) ion (Pb2+) and two chloride ions (Cl-). When you put these blocks in water, the water molecules are like little hands that try to pull them apart.
For the most part, these blocks are pretty tough. They like to stick together. So, only a few of them actually get pulled apart by the water.
The blocks that do get pulled apart split into their individual ions: one lead(II) ion and two chloride ions. These free-floating ions are now happily swimming around in the water.
The Equilibrium Shuffle
But here's the really cool part. It's not a one-way street! While some PbCl2 is dissolving, the ions that are already swimming around can also decide to get back together.
They find each other and reform the solid PbCl2. It’s like a constant, tiny back-and-forth dance happening at the molecular level. This is called equilibrium, and it's a fundamental concept in chemistry that's surprisingly engaging.
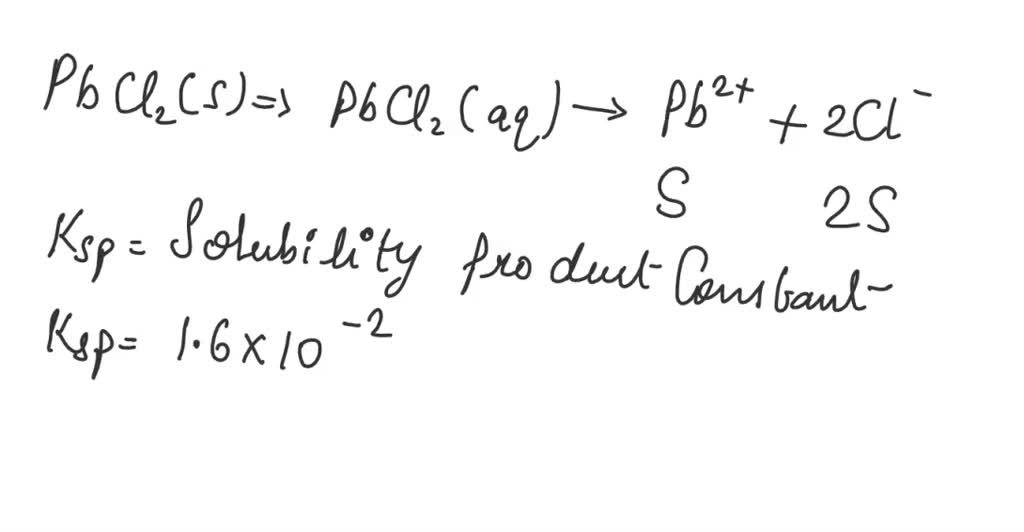
So, you have solid PbCl2 trying to dissolve, and dissolved lead(II) ions and chloride ions trying to recombine. They reach a point where the speed of dissolving and the speed of reforming are exactly the same. It looks like nothing is happening, but there's a whole lot of activity going on!
Introducing the Solubility Product Constant (Ksp)
Now, how do we describe this delicate balance? Scientists, being clever folks, came up with a special way to measure it. They call it the Solubility Product Constant, or Ksp for short.
Think of Ksp as a score that tells us just how much of a substance, like PbCl2, can dissolve in water at a specific temperature. A high Ksp means it dissolves a lot, and a low Ksp means it doesn't dissolve much at all.
For PbCl2, the Ksp is relatively low. This tells us it's not super soluble; most of it will remain as a solid, even if you add a lot of water.
The Grand Expression!
So, what's the actual expression for this mysterious Ksp for PbCl2? It's like writing down the recipe for that equilibrium dance.

When PbCl2 dissolves, it breaks apart into Pb2+ ions and Cl- ions. The expression for the Ksp is simply the product of the concentrations of these dissolved ions.
Here's where it gets a little bit "mathy," but in a fun, descriptive way! For every one unit of PbCl2 that dissolves, you get one lead(II) ion and two chloride ions. The "two" is important because it comes from the chemical formula of PbCl2.
The expression for the solubility product constant for PbCl2 is:
Ksp = [Pb2+][Cl-]2
Let's break that down.
Ksp is our score for how much dissolves.
[Pb2+] represents the concentration of the dissolved lead(II) ions. Think of this as how many of those lead pieces are swimming around freely.
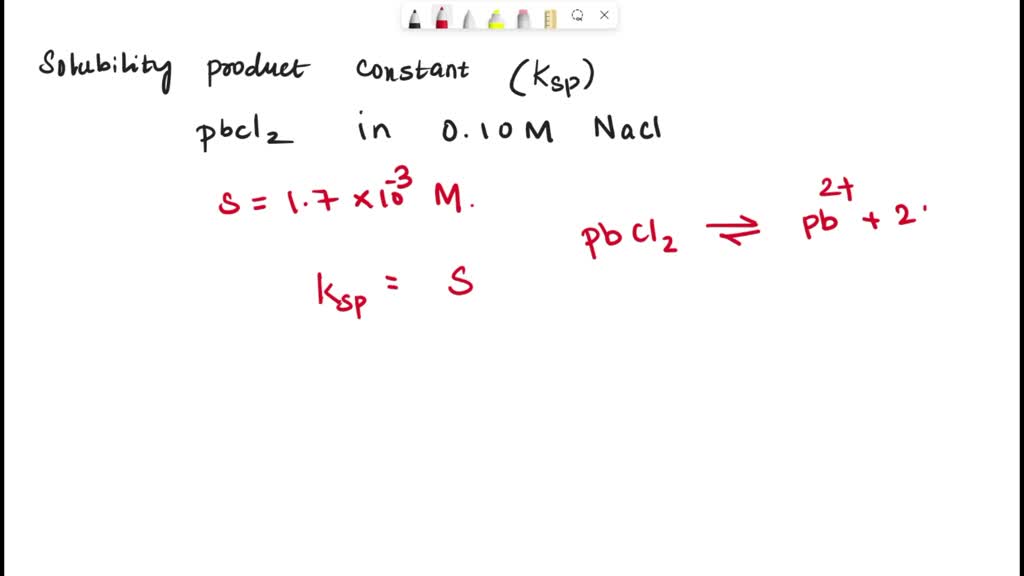
[Cl-] represents the concentration of the dissolved chloride ions. And the little "2" next to it? That's crucial!
The 2 means we have to multiply the concentration of chloride ions by itself. This accounts for the fact that you get two chloride ions for every one lead ion when PbCl2 dissolves.
Why is this so Entertaining?
You might be thinking, "Okay, that's a formula. What's so fun about that?" Well, the fun is in what this formula tells us!
It's like a secret code that explains the behavior of substances in water. This single expression, Ksp = [Pb2+][Cl-]2, unlocks mysteries about how much solid will precipitate out or how much can dissolve under different conditions.
It's the underlying principle for many everyday phenomena, from water purification to how medicines dissolve in our bodies. It's a tiny window into a vast and intricate chemical world.

What Makes it Special?
What makes this particular expression special for PbCl2 is its demonstration of how the stoichiometry (the way atoms are arranged in a molecule) directly impacts the solubility constant. The fact that there are two chloride ions for every lead ion isn't just a chemical fact; it's baked into the Ksp formula with that exponent.
It highlights that even seemingly simple compounds have complex behaviors governed by elegant mathematical relationships. This expression is a key to understanding the subtle but significant differences in how various salts behave in water.
It's a constant reminder that behind the scenes of the visible world, there are invisible forces and interactions shaping everything around us. And the Ksp for PbCl2 is just one little piece of that amazing puzzle.
A Little Bit of Chemical Detective Work
Imagine you're a detective investigating a cloudy solution. By knowing the Ksp for PbCl2, you can deduce a lot. Is there too much lead or too much chloride present? Is the solution saturated or undersaturated?
This simple expression allows chemists to predict and control chemical reactions. It’s like having a superpower to understand and manipulate the microscopic world.
So, the next time you see something dissolve or not dissolve in water, remember the tiny dance of ions and the elegant expression that describes it. The Solubility Product Constant for PbCl2 is more than just a formula; it's a story of balance, interaction, and the fundamental laws of chemistry.
