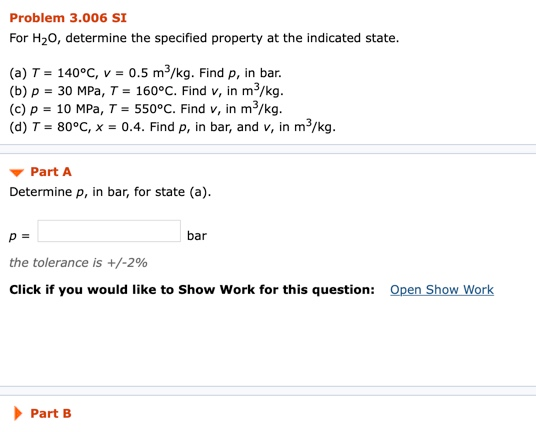
For H2o Determine The Specified Property At The Indicated State
Ever looked at a puddle, a steaming kettle, or a giant ice cube and just thought, "Wow, water is neat"? Well, get ready to have your mind blown (in a super chill, totally relaxed way, of course). We're about to go on a little adventure to understand what makes H2O, our favorite liquid friend, do its thing.
Think of H2O like a shape-shifter. It's the same stuff, whether it's a solid block of ice keeping your fancy drinks frosty or a wispy cloud floating in the sky. The big secret? It all comes down to the temperature and the pressure. Yep, those two things are like the directors of water's amazing play.
Imagine water is at a party. When it's feeling pretty mellow and cool, it likes to hang out in a nice, orderly fashion. This is when we see it as a solid – ice! It’s all structured and neat, like people standing in a perfectly straight line.
But then, the music picks up! When you crank up the heat, those water molecules get all excited. They start bouncing around, bumping into each other, and losing their neat structure. This is when water decides to become a liquid, just like the stuff in your tap or the rain falling on your window.
And if the party gets really wild, with lots of energy and space to move, the water molecules go absolutely bonkers! They zoom around, completely free, and spread out everywhere. This is the gas phase, also known as steam or water vapor. It’s like everyone at the party suddenly decided to do the robot dance.
Now, let's talk about the property that changes. Think about how easily water flows. When it's a liquid, it's pretty easy-breezy, right? You can pour it, splash it, and it does what you want. That's its viscosity at play.
When water is nice and cold, as ice, it’s not going anywhere! It’s solid, firm, and definitely not flowing. So, its viscosity in that state is, well, practically non-existent. It’s like trying to pour a brick – not happening.
As water warms up and becomes a liquid, its viscosity starts to show. It has a certain "thickness" or resistance to flow. Some liquids are thick like honey (high viscosity), while others are thin like juice (low viscosity). Water is somewhere in the middle, making it perfect for most of our daily needs.

And when water turns into steam, it’s so spread out and energetic that it’s not really "viscous" in the way we think of liquids. It’s more like a gas, with very little resistance to movement. It just… floats away!
But what about when things get really weird? Imagine you’re at the top of a really, really tall mountain, where the air is thin. This is where pressure comes in. Pressure is like a crowd of people trying to squeeze into a small room.
When water is under high pressure, it gets a bit stubborn. It’s harder for those molecules to spread out. This means that even if you heat it up, it might not turn into steam as easily as it would at sea level.
Conversely, if you're in a place with super low pressure, like a fancy vacuum chamber (don't try this at home!), water can get a bit confused. It might start to boil and turn into steam even at pretty cool temperatures!
So, the same old H2O can be a rigid ice cube, a flowing drink, or an invisible puff of steam. It’s all about what kind of party the molecules are having, influenced by how much energy they have (temperature) and how much space they’re allowed to move around in (pressure).
Think about your morning coffee. When it's hot and steaming, the water molecules are practically dancing. That's H2O in its gaseous state, spreading its warmth and aroma.
Then, as it cools in your mug, it becomes the familiar liquid we love to sip. It has a lovely, smooth flow, ready to be enjoyed. This is the liquid state, where viscosity is just right.
And what if you accidentally left your coffee outside on a freezing night? You’d find a solid block of ice. No more flowing, no more steam. Just a firm, frozen block, a testament to water’s solid state.
It’s a beautiful dance of energy and space. This simple molecule, H2O, is constantly changing its tune, revealing different sides of its personality based on its surroundings.
It’s this incredible adaptability that makes water so vital to life. From the freezing glaciers of the poles to the steamy rainforests, water is always there, taking on the form that best suits its environment.
So, next time you see water in any of its forms, take a moment to appreciate the magic. It’s not just water; it’s H2O, the ultimate chameleon, expertly navigating the world of temperature and pressure to show us a new wonder with every change.
It’s a reminder that even the simplest things around us hold a universe of fascinating behavior. The way water behaves isn’t just science; it’s a grand, ongoing performance, and we get to witness it every single day.
Whether it's a crystal-clear ice sculpture, a refreshing glass of water, or the invisible moisture in the air, each state of H2O has its own unique charm and purpose.
The specific property we might be interested in, like how easily it flows (viscosity) or how much energy it takes to change its state (like boiling), is all determined by these two crucial factors: temperature and pressure.
It’s like water has a closet full of outfits, and temperature and pressure are the stylists helping it pick the perfect one for any occasion.
And the best part? It’s all happening around us constantly, a silent, amazing ballet of molecules.
So, the next time you drink a glass of water, or shiver in the cold, or feel the warmth of steam, remember you're experiencing H2O in its specified property at its indicated state.
It's a simple molecule, but its story is anything but. It's a story of transformation, adaptation, and the incredible power of physics at play in our everyday lives.
Embrace the wonder of water. It’s more than just a drink; it’s a testament to the dynamic, ever-changing nature of our amazing planet.
And who knows? Maybe next time, we'll explore how this same little molecule helps keep us all alive and kicking. That’s a whole other adventure!
