For Cis-1 3-dimethylcyclohexane Which Two Chair Conformations Are In Equilibrium
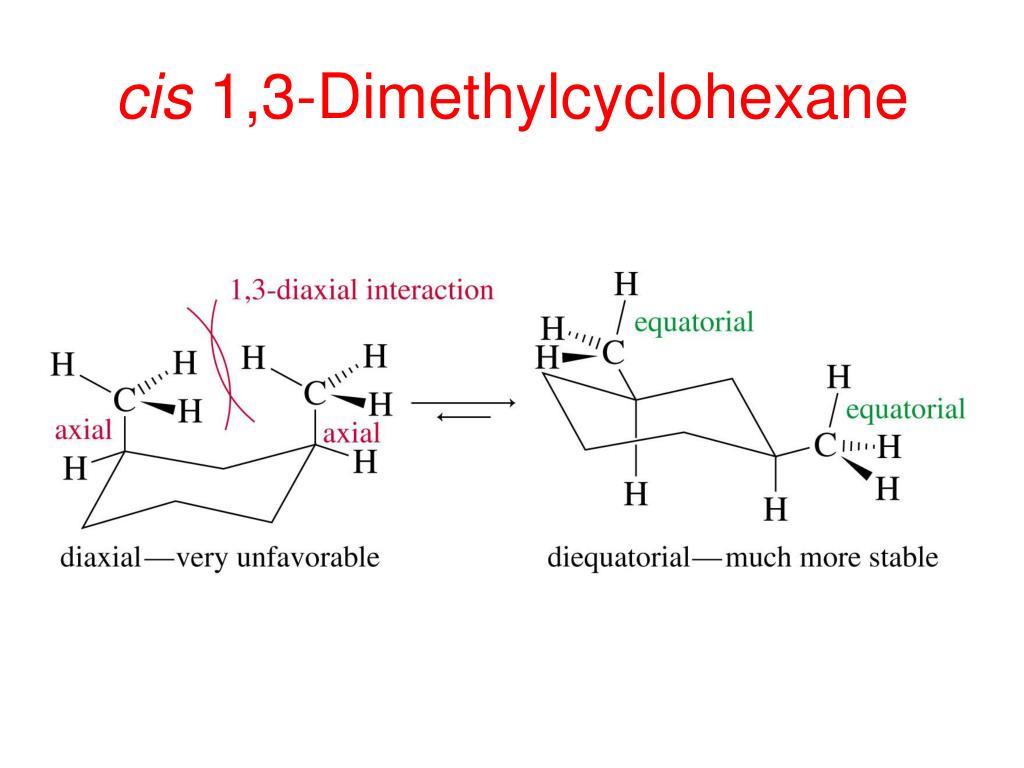
Hey there, science enthusiasts and curious minds! Ever feel like your favorite armchair just isn't quite as comfy as it used to be? You know, you shift a little, and suddenly it feels like a whole new throne? Well, get ready to have your socks knocked off, because molecules do something super similar, and today we're diving into the wild world of a certain cyclohexane buddy called cis-1,3-dimethylcyclohexane!
Now, cyclohexane itself is like a little ring of six carbon atoms, all holding hands. It’s got this amazing ability to twist and turn, sort of like a hula dancer with an extra-bendy spine. These twists aren't just for show; they're crucial for how the molecule behaves. Think of it like this: imagine you have a bicycle wheel. You can spin it, right? Cyclohexane is like that, but instead of spokes, it has atoms, and it can twist into different shapes, which we scientists lovingly call conformations. It’s like giving your hula hoop a little shimmy and shake to get it just right.
So, we’re zooming in on a specific cyclohexane. This one has two little methyl groups – think of them as tiny little "arms" sticking out from the carbon ring. These arms are attached to carbons number 1 and 3. And here's the kicker: they're on the cis side. What does cis mean? Imagine two friends standing next to each other on a street. If they're both on the same side of the road, they're 'cis'. If one's on one side and the other's on the opposite, they're 'trans'. So, for our cis-1,3-dimethylcyclohexane, these two methyl groups are BFFs, hanging out on the same side of that cyclohexane hula hoop!
Now, our cyclohexane ring is a bit of a shape-shifter. It loves to flip-flop between its most comfortable positions. These are its chair conformations. Why "chair"? Because when you look at it just right, it kind of resembles a comfy armchair, complete with a backrest and armrests. It’s got a superior seat and a rather relaxed leg situation. It’s the molecular equivalent of finding that perfect slouch!
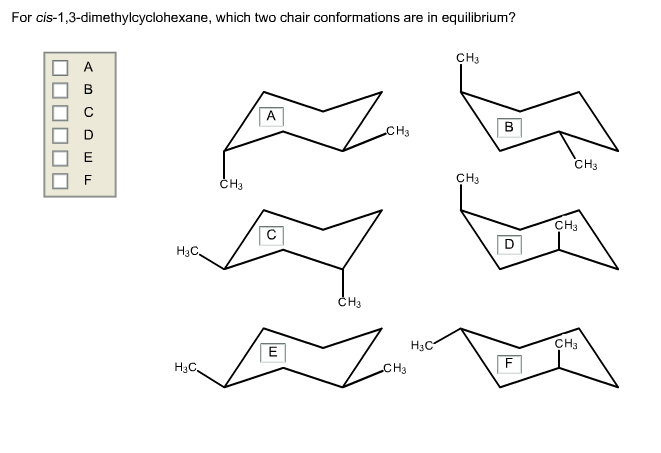
For most cyclohexanes, these chair conformations are like siblings – pretty similar, but one might be slightly more chill than the other. However, with our cis-1,3-dimethylcyclohexane, things get even more interesting! It has two chair conformations that are locked in a constant dance, a molecular tango, if you will. They’re in a state of glorious equilibrium. What’s equilibrium? Imagine you have a perfectly balanced scale. For every little thing that tips one side down, something equally important tips the other side up. It’s a dynamic, energetic balance, like a perfectly orchestrated juggling act. The molecules are constantly switching between these two shapes, but the overall picture stays the same. They're not getting stuck; they're just… switching!
So, what are these two superstar chair conformations that are having this never-ending molecular party? They are essentially mirror images of each other, but with a crucial twist in how those methyl groups are positioned. In one chair, one methyl group might be in a super chill, equatorial position – think of it as lounging on the widest part of the chair. The other methyl group is also in an equatorial position, but because of the 'cis' rule, they are on the same 'side' of the ring. Now, the other chair conformation is where the magic happens! The ring flips, and our little methyl groups, still on the same side of the ring, find themselves in slightly different orientations. One might still be equatorial, but the other, due to the ring flip, also ends up in a wonderfully relaxed equatorial spot! It’s like finding two equally amazing spots on the couch to binge-watch your favorite show.
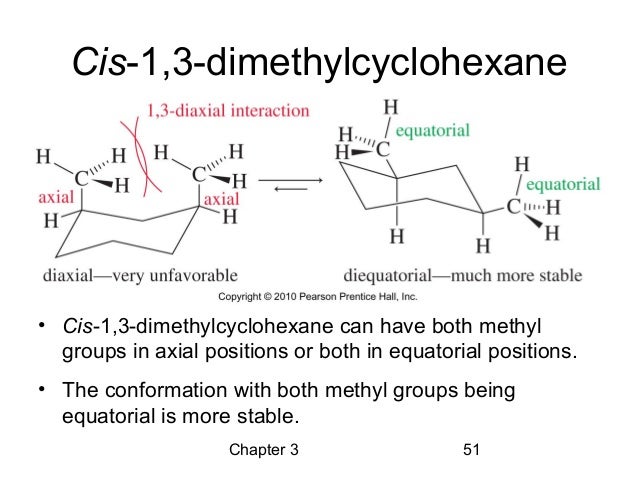
The beauty of this is that because both methyl groups are on the same side of the ring (the 'cis' part), when the cyclohexane ring does its signature flip, it creates two very, very similar, and therefore equally stable, chair conformations. It’s like having two identical prize-winning cookies; you can’t decide which one is the best because they’re both perfection! These two chair conformations are so close in energy, so perfectly balanced, that they exist in a delightful state of equilibrium. The molecule is just having a blast, continuously morphing between these two equally fabulous lounging positions. It’s not a struggle; it’s a celebration of molecular flexibility!
So, the next time you’re adjusting your pillow for that perfect night’s sleep, give a little nod to cis-1,3-dimethylcyclohexane. It’s out there, in its own tiny molecular world, doing its own amazing chair-flipping dance, finding equilibrium in two equally comfy conformations. It’s a small example, but it’s a fantastic reminder that even at the smallest scales, nature is full of wonder, balance, and a whole lot of fun!
