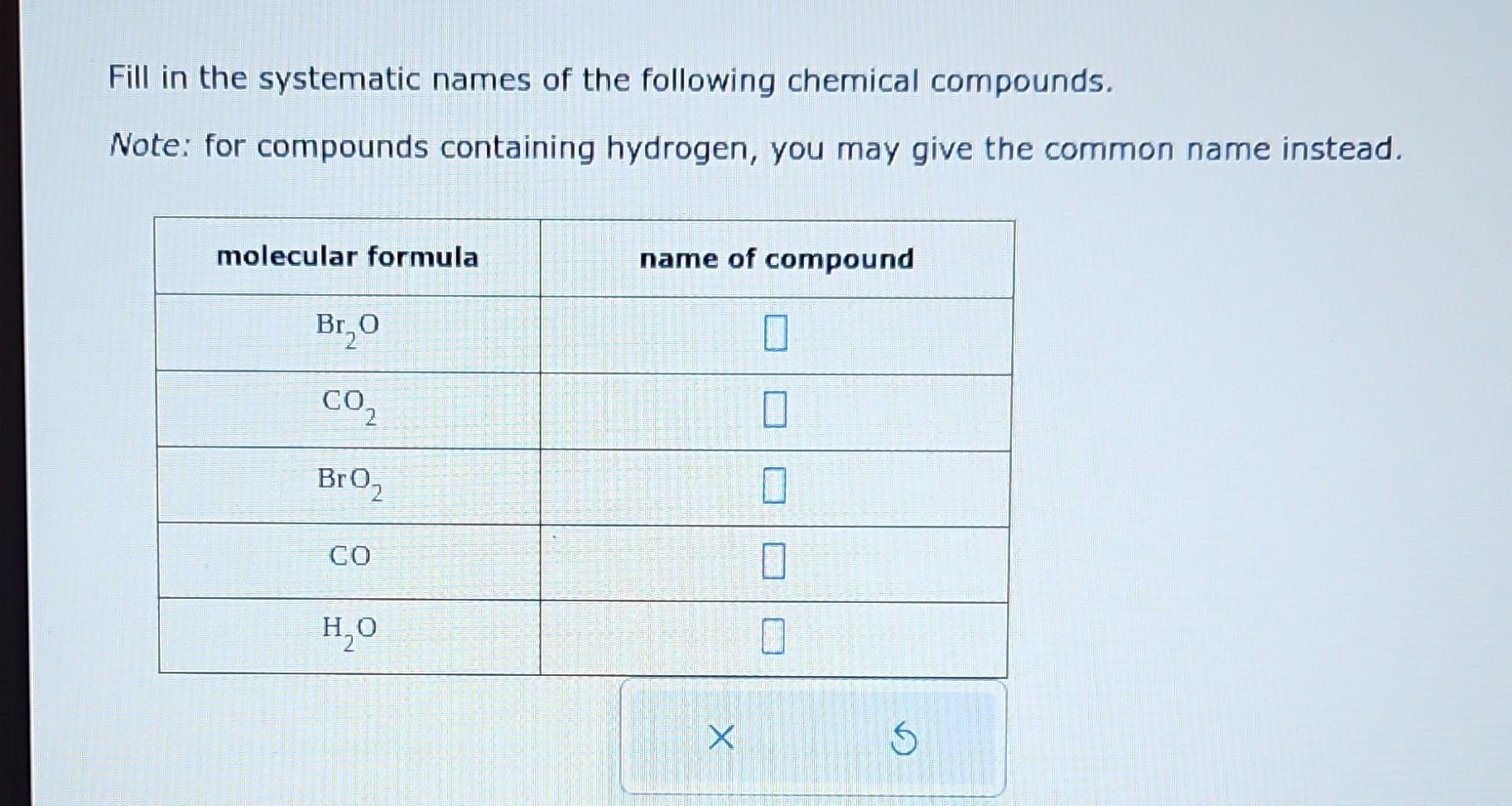
Fill In The Systematic Names Of The Following Chemical Compounds

Okay, let's talk about something that might make your brain do a little jig. We're diving into the wonderfully wacky world of chemical names.
You know, the ones that sound like they were invented by a committee of overly enthusiastic scientists with too much coffee.
It’s like they took a perfectly normal molecule and said, "How can we make this sound like a secret code only wizards can crack?" And then, poof! A new, impossibly long name was born.
I've got a few of these linguistic marvels here, and honestly, my brain just wants to put on tiny little earmuffs and hum a happy tune.
But we're going to tackle them, right? For science! Or, you know, just for a good chuckle.
Here are a few brave souls that have volunteered for our naming adventure. Your mission, should you choose to accept it (and you kind of have to, you're reading this), is to fill in the blanks with their proper, fancy-pants systematic names.
The Trials and Tribulations of Nomenclature
Sometimes, I suspect these names aren't just for identifying substances. Maybe they're a secret test.
A test to see if you've truly mastered the art of staring blankly at a string of letters until your eyes cross.
And don't even get me started on the prefixes and suffixes. They're like tiny linguistic ninjas, sneaking in and changing the whole meaning.
Is it a "di-" something? Or a "tri-" something? Are we talking about a "heptane" or a "hexane"? My inner elementary school student is already hiding under the desk.
It's like a verbal obstacle course. You hop over "eth-", dodge "meth-", and try not to get tangled in a "phenyl."
And the numbers! Oh, the numbers. They’re just there, in the middle of it all, like little punctuation marks that decide the fate of the entire name.
So, without further ado, let's see if we can coax these chemical critters out of their hiding spots.
Compound 1: The Speedy One
Our first contender is a classic. It's the kind of thing you might find in, well, lots of things.
Think of it as the reliable friend of the chemical world. Always there, doing its job.
When you see its structure, it just screams simplicity. A nice, neat row of carbon atoms with some friends attached.
But its name? It’s a little more… enthusiastic. It’s like it wants to be heard from across the room.
This one is all about a chain. A specific number of carbons, to be precise.
And the ending tells you exactly what kind of atoms are holding hands. It's a real tell-tale sign.
It's a simple hydrocarbon, but its name has a certain… flair. A flair that might involve a bit of head-scratching.
Let's give it a proper introduction. We’re looking for the systematic name of:
A hydrocarbon with a chain of 6 carbon atoms.
What do you think it is? Does the number 6 ring any bells? Does the ending sound like something you’d find at the end of a long line?
My guess is that if you think of a common gas used for lighter fluid, you might be on the right track. Or perhaps a solvent.
The systematic name for this particular arrangement of carbon and hydrogen is a single, elegant word. No fuss, no muss, just pure chemical identification.
So, what is it? The answer, my friends, is a rather straightforward: ____________________.
Compound 2: The Branched Mastermind
Now, things get a little more interesting. Our next compound isn't just a straight line.
It's decided to be a bit more adventurous, branching out and exploring new possibilities. Like a tiny chemical rebel.
This one has a main chain, yes, but it also has a little side trip. A small group of atoms hanging off the main route.
Identifying these requires a bit of a detective's mind. You have to find the longest chain first. That's your foundation.
Then, you have to figure out what's attached to that chain and where it's attached. The position matters, you see.
It’s like giving directions to a very specific place. "Turn left at the big tree, then take the third exit."
This compound features a main chain of 5 carbon atoms. But there's a methyl group (that's one carbon with three hydrogens) attached to the second carbon of that chain.
So, we have a chain and a little helper. How do we put that together into one coherent, yet utterly intimidating, name?
![[SOLVED] Fill in the systematic names of the following chemical](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/12/6398d07cc4fcc_2286398d07ca09b9.jpg)
The rules of systematic naming are your guide here. Start with the longest chain, then add your substituents.
The substituent gets a prefix indicating its size and an "-yl" ending. And its location is crucial.
It’s a bit like assigning a nickname that also tells you where the person lives. "Little Johnny from the corner house."
We need to combine the position, the substituent name, and the parent chain name.
The systematic name for this strategically branched molecule is: ____________________.
Compound 3: The Oxygen Lover
Alright, let's introduce some new friends into the mix. This next compound involves the ever-important element: oxygen.
Oxygen is like the social butterfly of chemistry. It loves to bond with everyone.
In this case, oxygen is bridging two carbon chains. It’s like the connector in a tiny molecular friendship bracelet.
This is where things start to get a little more descriptive. The name needs to tell us about this bridging oxygen.
We have two ethyl groups. Remember those? Two carbons each.
And these two ethyl groups are linked together by a single oxygen atom. Think of it as di-ethyl ether, but we need the fancier systematic name.
The systematic naming convention for these types of compounds often involves using "alkoxy" prefixes.
"Alkoxy" basically means an alkyl group (like ethyl) attached to an oxygen. So, we have an ethoxy group.
Then, this alkoxy group is attached to another alkyl group. In this case, another ethyl group.
It's about identifying the "main" chain and then describing the "branch" that includes the oxygen.
You're looking for a name that describes the structure clearly, even if it sounds like a tongue twister.

The systematic name for a molecule where an oxygen atom connects two ethyl groups is: ____________________.
It's a bit of a mouthful, isn't it? But oh-so-precise.
It’s like the chemical equivalent of a perfectly tailored suit. Every part is in its right place.
Compound 4: The Double Bond Dancer
Now, we're adding a little more excitement. This molecule has a double bond between two carbon atoms.
Double bonds are like little energetic sparks in a molecule. They change its reactivity and its properties.
This compound is a hydrocarbon with a chain of 5 carbon atoms, and there's a double bond between the first and second carbon atoms.
The presence of a double bond changes the ending of the name. It's no longer just an "-ane" situation.
It becomes an "-ene." A subtle but significant shift.
And, just like with the branched compound, the position of the double bond is crucial information.
We need to indicate where this double bond makes its grand entrance.
So, we have a 5-carbon chain, a double bond at the beginning, and all the rest are hydrogens filling in the gaps.
Think of it as numbering the carbons and then pointing out the location of the party happening between carbons 1 and 2.
The systematic name for this 5-carbon hydrocarbon with a double bond starting at the first carbon is: ____________________.
It’s a name that elegantly communicates the structure and reactivity.
It’s a dance of atoms, and the name tells us the steps.

Compound 5: The Ring Leader
Finally, let's enter the realm of cyclic compounds. This one forms a ring!
Instead of a straight chain, the carbon atoms have decided to hold hands in a circle. A molecular huddle.
This particular ring is made up of 6 carbon atoms, all connected in a perfect hexagon.
And each of these carbon atoms is bonded to a single hydrogen atom. It's a very symmetrical arrangement.
The prefix for a ring structure is usually "cyclo-". It clearly signals that we're dealing with a closed loop.
Then, you add the name of the parent hydrocarbon based on the number of carbon atoms in the ring.
So, if you have a 6-carbon ring, and you know the name for a 6-carbon straight chain, you're most of the way there.
It's like giving a piece of jewelry a name: a six-sided ring.
The systematic name for a ring structure containing 6 carbon atoms is: ____________________.
There you have it! A little journey into the delightful, and sometimes daunting, world of chemical names.
Did you conquer them? Did your brain do a little happy dance or a confused wobble?
Either way, you faced the systematic names and, dare I say, came out victorious.
It's a language of its own, and while it might seem complex, it's wonderfully precise.
So next time you see a long chemical name, just remember the fun we had trying to decode it.
And maybe, just maybe, you’ll crack a smile. Or at least nod in understanding.
Keep exploring, keep questioning, and keep filling in those blanks!
