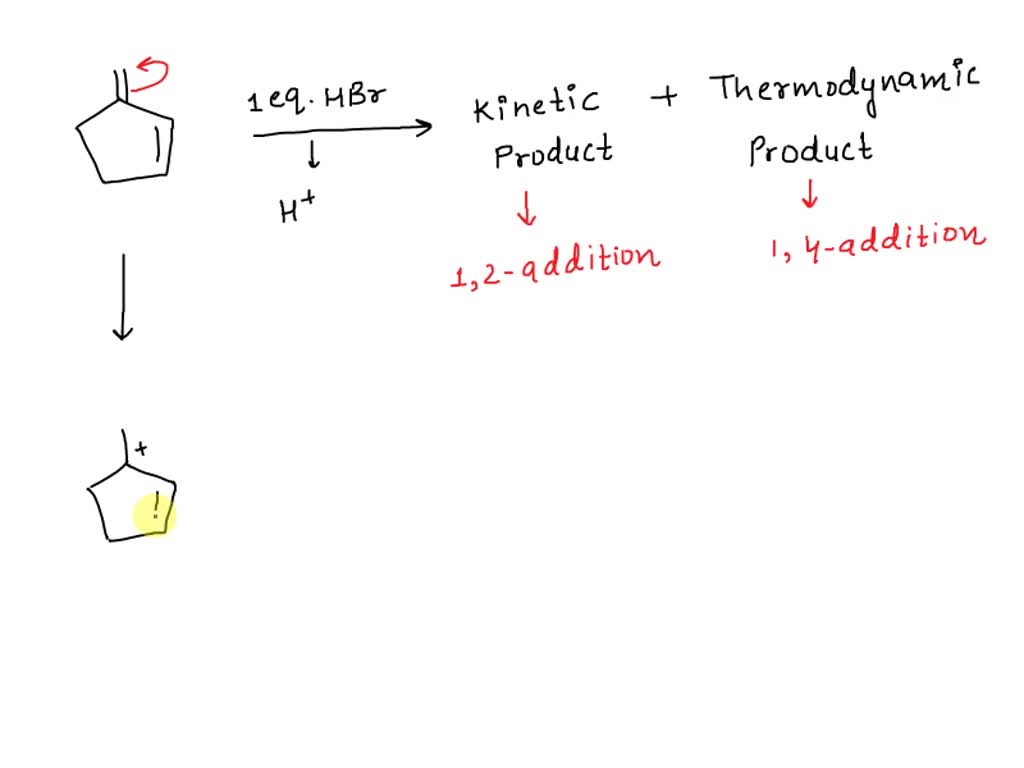
Draw The Major Thermodynamic And Kinetic Products Of The Reaction.
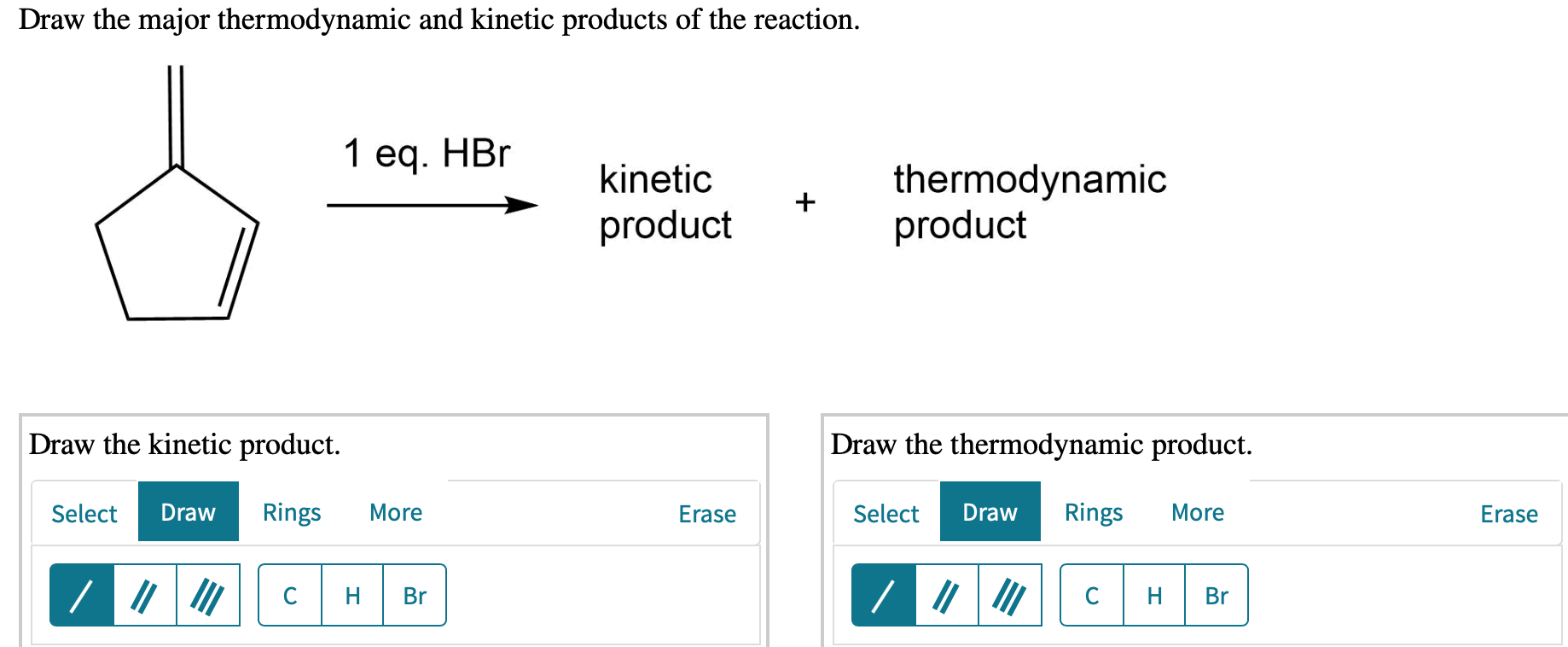
So, you’ve stumbled upon this whole "thermodynamic and kinetic products" thing, huh? Don't worry, it's not some ancient secret society initiation or a recipe for the perfect soufflé (though, honestly, soufflés have a lot in common with this). Think of it like this: ever been in a situation where you had a couple of options, and one was super quick and easy, but maybe not the best long-term, and the other took a bit more effort but ended up being way more satisfying? Yep, you've been living the thermodynamic and kinetic life without even realizing it!
Let's break it down, but in a way that doesn't involve squinting at a whiteboard covered in squiggly lines and Greek letters. Imagine you're trying to get from your couch to the fridge. Simple, right? But there are ways to do it. You could, say, just kind of… roll there. It’s fast, requires minimal effort, and gets you to that sweet, sweet beverage. That's your kinetic product. It’s the low-hanging fruit, the path of least resistance, the route you take when you’re really hungry and can't be bothered to stand up too straight.
Now, imagine a slightly more ambitious journey to the fridge. You could get up, walk with purpose, maybe even do a little jaunty skip. This takes a tiny bit more energy, a smidge more coordination. But at the end, you're not just at the fridge, you’re standing there, feeling a little more… accomplished. You’ve arrived in a more stable, perhaps even more comfortable, position. This is your thermodynamic product. It's the "cooler, more stable" option, the one that’s going to be just right once you get there, even if it took an extra few seconds to achieve.
In the wild world of chemistry, these "paths" are like the different ways a molecule can rearrange itself. When you throw some reactants together and give them a little nudge (heat, light, whatever gets them going), they’re like little eager beavers, just itching to change. They’ll explore all sorts of possibilities, like a toddler discovering new ways to stack blocks. Some of these rearrangements happen super fast. They’re the immediate gratification, the "ooh, shiny!" reactions. These are your kinetic products.
But here’s the kicker: sometimes, the fastest way isn’t the best way. It might be a bit wobbly, prone to falling apart, or just… not quite what you were hoping for in the long run. These are the kinetic products that might be a bit like that trendy outfit you bought that looked amazing in the store but is a nightmare to wear for more than an hour. They're here for a good time, not a long time.
The thermodynamic product, on the other hand, is like your trusty old comfy sweater. It might not have been the first thing you grabbed, and it might have taken a little more effort to find (or, in chemistry, to form), but it's where the molecules really want to settle down. It's the most stable arrangement, the one that’s going to stick around. Think of it as the happy ending, the place where everyone sighs contentedly and says, "Ah, that's more like it."
So, How Do We Know Which is Which?
This is where the magic (and a bit of science) happens. Think of it like a party. You've got a bunch of guests (molecules) mingling. Some are going to be drawn to the loudest music and the fastest dancing – that's the kinetic crowd. They’re having a blast right now. Others might be having a more relaxed conversation in a quieter corner, enjoying the ambiance and the good company – that’s the thermodynamic crowd.
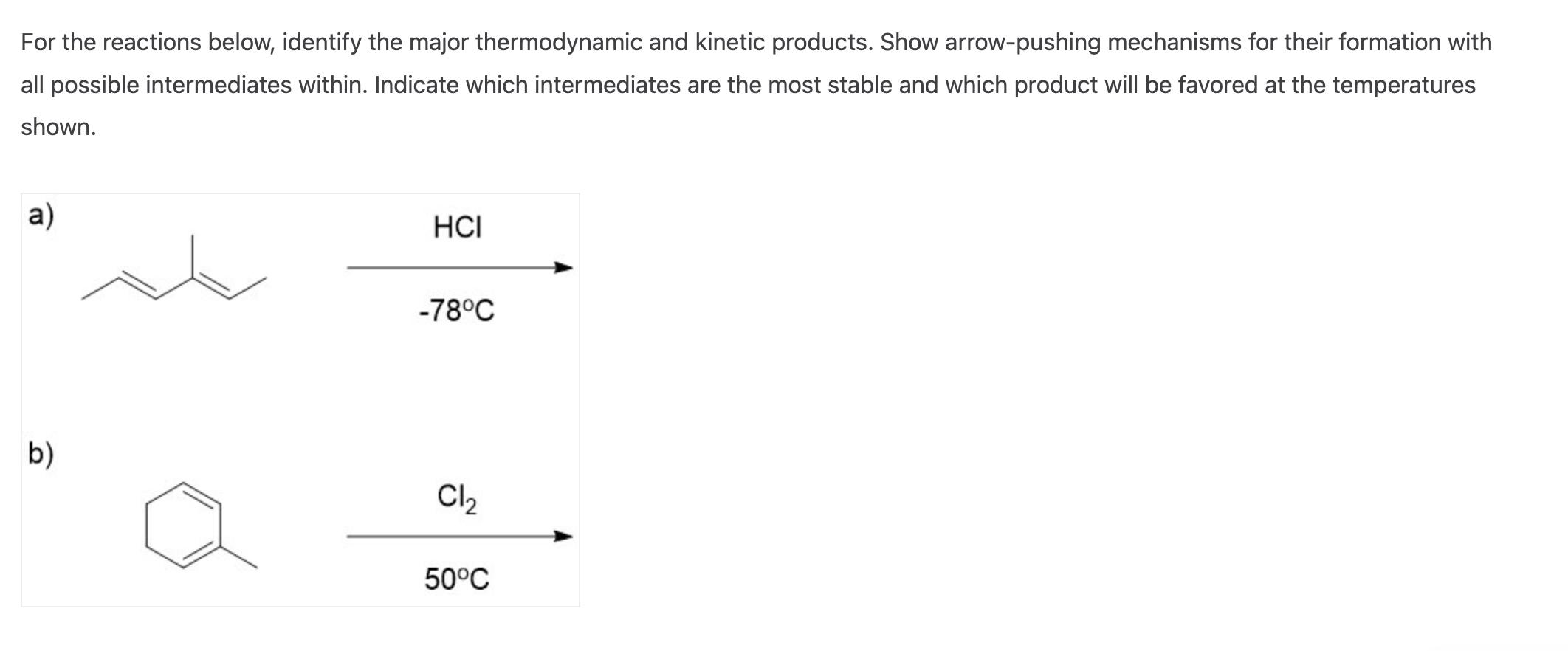
Now, if the party is short and loud, you're going to see a lot more of the kinetic dancers. They're the first ones to show up and the last ones to leave the dance floor. But if the party goes on for a really long time, and maybe there’s some really good food and comfortable seating, those quieter conversationalists might eventually outnumber the dancers. They’ve had time to find their groove, to settle into the most comfortable spots. This is like the effect of temperature and reaction time in chemistry.
Low temperatures? Think of it as a quick, energetic party. You don't have a lot of time for folks to change their minds or find the "best" spot. So, whatever happens first, whatever is the easiest to achieve, that’s what you’re going to get. That’s your kinetic control. It's like slamming on the brakes in your car – the car stops, but it might skid a bit. It’s effective now, but maybe not the smoothest maneuver.
High temperatures? Now we're talking about a long party with plenty of time for everyone to socialize, find the best snacks, and discover the comfiest sofas. If you give the molecules enough time and energy (heat!), they can rearrange themselves. They can undo what they just did and try a different path, a potentially better, more stable one. This is thermodynamic control. It’s like gently easing into a parking spot. It takes longer, but you end up perfectly aligned and stress-free.
Imagine you're baking cookies. You mix up the dough, and you can bake them right away. Those cookies will be done quickly, but maybe they'll be a bit soft in the middle, or not quite as spread out as you’d like. That’s your kinetic product. But if you let the dough rest in the fridge overnight (giving it time and a slightly cooler, but still energetic, environment), the butter will firm up, the flavors will meld. When you bake that dough, you might get a slightly different, arguably better, cookie. That's your thermodynamic product.
![[GET ANSWER] predict the major product for the reaction shown here and](https://cdn.numerade.com/ask_images/801edbd124e04b6d8093f6e548b3623e.jpg)
Let's Get a Little Sketchy (But Not Too Sketchy)
Okay, so when you're actually drawing these things, it's like drawing a little map of how the molecules can travel. We often use something called a "reaction coordinate diagram." Don't let the fancy name scare you. It's basically a graph that shows the energy levels of the molecules as they transform.
Think of it like a rollercoaster. The starting point is where your reactants are, chilling at their initial energy level. Then, as they start to react, they have to climb over an "energy hill" to get to the next stage. This hill is called the activation energy. It's like that little burst of energy you need to get off the couch and start doing something.
The kinetic product is the one that’s reached via the shortest or lowest hill. It's the quick win. You get over that little bump, and boom, you're there! It’s formed quickly, but it might be sitting in a slightly higher energy valley. Think of it as a slightly unstable perch on the rollercoaster.
The thermodynamic product is the one that’s in the lowest energy valley overall. It might have a higher initial hill to climb (a bigger activation energy), meaning it takes more effort to get to it. But once the molecules get there, they are super happy and stable. They're at the bottom of a nice, deep canyon, not going anywhere in a hurry.
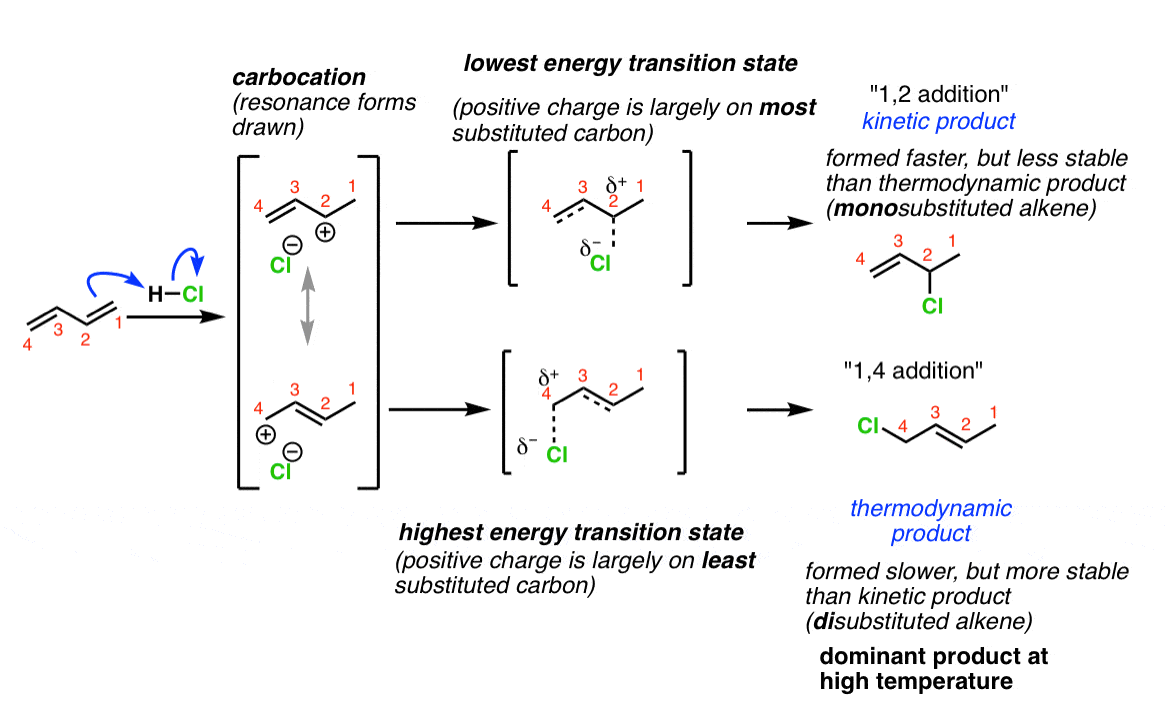
So, on your diagram, you'll have your starting reactants. Then, you'll have a hump (the transition state) leading to the kinetic product. This hump is usually the lower one if the kinetic product is favored. Then, there might be another hump, possibly higher, leading to the thermodynamic product. This product will be at a lower energy level than the kinetic product.
Imagine you’re trying to get from your house to two different friends’ houses. Friend A lives just around the corner, a quick 5-minute walk. That’s your kinetic product – easy, fast, right there. Friend B lives across town, and the direct route involves climbing a pretty steep hill. It takes 30 minutes. But once you get to Friend B's, you have a fantastic view, comfy seating, and amazing snacks. That's your thermodynamic product – takes more effort, but it's ultimately more rewarding and stable.
The key is that the molecules can go back and forth. If the kinetic product is formed quickly, but it’s in a higher energy valley, it has enough energy to climb back up the hill and try again. If it has enough time and energy (especially at higher temperatures), it will eventually make its way to the lowest energy valley, the thermodynamic product. It's like realizing your quick trip around the block isn't as satisfying as that long hike to the beautiful viewpoint, so you decide to go back and do the hike instead.
Real-World Analogies are Our Best Friends
Let’s talk about something we all know: dating. You meet someone, and there's instant chemistry! You’re laughing, you’re having fun, everything’s easy. That’s a kinetic relationship. It’s exciting, fast-paced, and often built on immediate attraction. It’s the “love at first sight” situation.
Now, sometimes, that initial spark fizzles out because there aren’t deeper foundations. It was fast, but not necessarily built to last. That’s like a kinetic product that’s a bit unstable. It’s there, but it might not be there for long.
Then there are those relationships that start off a little slower. Maybe you don’t have that immediate fireworks display. It takes time to get to know each other, to build trust, to navigate the inevitable bumps in the road. But over time, you realize you’re deeply compatible, you support each other, and you have a really solid, enduring connection. That’s your thermodynamic relationship. It took more effort to build, but it’s stable, resilient, and ultimately much more satisfying.
The “thermodynamic” relationship is the one that has the most stable long-term potential. It might not have been the quickest to get to, but it’s the one that’s going to weather storms and bring lasting happiness. The “kinetic” relationship is the one that forms fastest, the one that’s easy to get into, but might not have the same staying power.
In chemistry, it’s the same principle. We’re looking for the most stable arrangement of atoms. Sometimes, the quickest way to get an arrangement isn’t the most stable arrangement. But with enough time and a bit of a push (heat!), the molecules can rearrange themselves into their ultimate happy place, the thermodynamic product.
So, next time you’re faced with a choice, whether it’s deciding on dinner or tackling a tough chemistry problem, remember the kinetic versus thermodynamic dance. Is it the quick, easy fix, or the more deliberate, rewarding outcome? Sometimes, the fastest route is fine, especially if you’re just grabbing a quick snack. But for those bigger, more important decisions, the thermodynamic approach often wins in the long run. And that, my friends, is the beauty of how things work, both in our kitchens and in our flasks!
