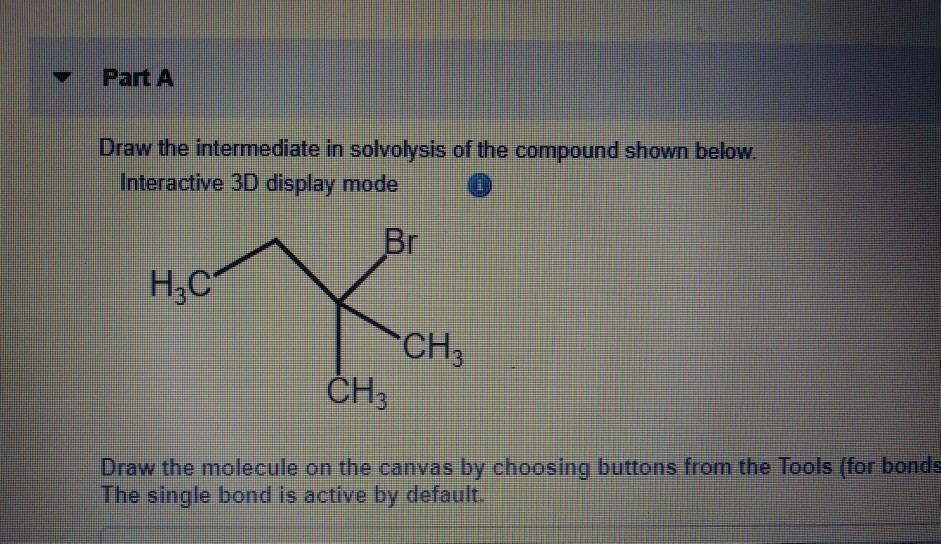
Draw The Intermediate In Solvolysis Of The Compound Shown Below.
Okay, let's dive into the wonderfully bizarre world of chemistry. Sometimes, when we're looking at molecules doing their thing, things get a little... interesting. It's like a chemical drama unfolding, and we're just here for the popcorn. Today, we're going to peek behind the curtain at a specific molecular moment. Think of it as a sneak peek at a movie trailer, but for molecules.
We're talking about solvolysis. Sounds fancy, right? It basically means a molecule is getting a bit of a shake-up, thanks to a solvent. Solvents are the unsung heroes of chemistry, aren't they? They're like the background actors that make the whole production possible. Without them, our molecules would just be sitting there, looking pretty, but not doing much. And let's be honest, we want them to do things.
Now, the particular molecule we're focusing on is… well, it's got a bit of flair. It's not just a simple straight line of atoms. It's got some curves, some bends, some personality. And when this particular molecule decides to undergo solvolysis, things can get a little complicated. It’s not always a clean break, you know? Sometimes, it’s more like a messy divorce.
During this solvolysis adventure, our molecule doesn't just magically transform into something new. Oh no. There’s a middle child. A stage in between. An intermediate. This is the character that shows up for a scene or two, causes a little bit of drama, and then exits, making way for the final product. These intermediates are like the plot twists in our molecular story. You think you know where it's going, and then BAM! Something totally unexpected pops up.
So, what does this intermediate look like? This is where things get a little… artistic. Imagine you have a molecule that’s kind of clinging to its parts. It’s thinking about breaking apart, but it’s not quite there yet. It’s in that awkward phase, where bonds are stretching, and new ones are thinking about forming. It's like when you’re trying to decide if you should commit to that second slice of cake. You’re hovering, contemplating, the decision is not yet made.
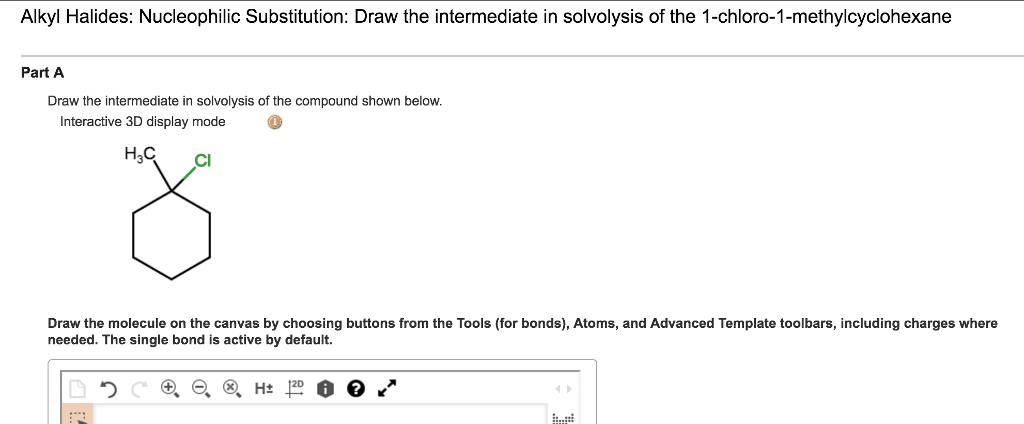
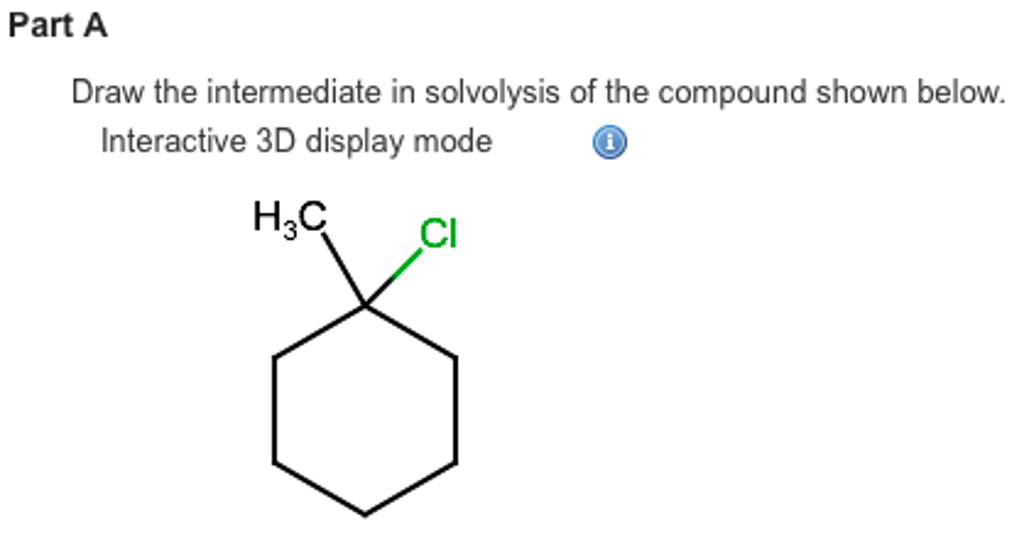
For our specific compound, when it’s chilling out in its solvent, waiting for the solvolysis party to get started, it’s going to do something rather… flamboyant. It’s going to create a situation where a positive charge appears. Now, positive charges in molecules are like the attention-seekers. They want to be noticed. They want to interact. And this positive charge isn't just going to sit quietly in a corner. It’s going to be pretty darn mobile. It's going to want to explore its options. Think of it as a molecule on a dating app, swiping left and right, looking for its perfect match to bond with.
The really fun part is how this positive charge likes to move around. It's not a one-and-done deal. It's more of a travelling circus of positivity. It can hop from one atom to another. This is where the term carbocation comes in. Yes, it sounds like a fancy new robot butler, but it's actually just a carbon atom with a positive charge. And this carbocation is the star of our intermediate show. It’s the reason why the whole solvolysis process gets interesting.
So, as our molecule starts its solvolysis journey, it’s going to shed a bit of electron-loving goodness, leaving behind a positively charged carbon. But this isn't a stable situation. It's like trying to balance a broom on your finger. It requires constant adjustment. And our molecule is going to adjust. It’s going to rearrange itself to make that positive charge more comfortable. It might even get a little help from its friends (other atoms in the molecule) to stabilize itself.
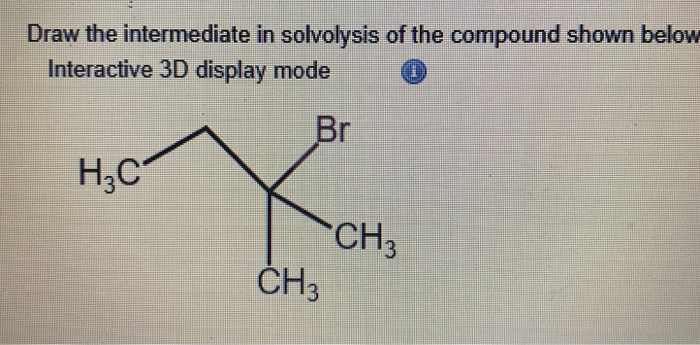
The key is that this intermediate is a fleeting moment. It’s here, then it’s gone, making way for the final, stable product. But in that brief window, it dictates the entire reaction pathway. It’s the director of the molecular movie. And the structure of this intermediate, this transient carbocation, is what we're drawing. It's a snapshot of a molecule in flux. It's a moment of beautiful chemical chaos.
Sometimes, the most interesting parts of a process are the bits that don't last very long.
Solved Part A Draw the intermediate in solvolysis of the | Chegg.com
So, when you’re looking at the drawing of this intermediate, don’t just see atoms and bonds. See the drama. See the decision-making. See the molecule bravely venturing into a temporary, charged state, all in the name of getting to its ultimate destination. It’s a chemical adventure, and this intermediate is the thrilling, albeit brief, climax before the resolution.
It's like when you're baking a cake. The batter is the starting material. The baked cake is the final product. But that moment in the oven, where it’s rising and transforming? That’s the magical intermediate stage. And for our molecule, this positively charged carbocation intermediate is that magical, slightly precarious, moment.
So, there you have it. A little peek into the life of a solvolysis intermediate. It’s not always straightforward, but it’s always fascinating. And honestly, who doesn't love a good chemical plot twist?